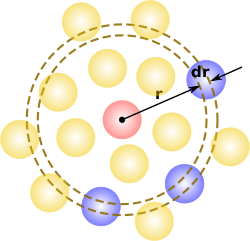
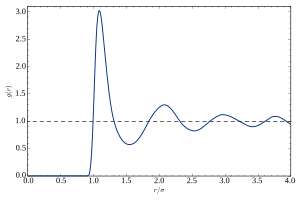
Radial distribution function
), describes how density varies as a function of distance from a reference particle.
In simplest terms it is a measure of the probability of finding a particle at a distance of
The radial distribution function is usually determined by calculating the distance between all particle pairs and binning them into a histogram.
Given a potential energy function, the radial distribution function can be computed either via computer simulation methods like the Monte Carlo method, or via the Ornstein–Zernike equation, using approximative closure relations like the Percus–Yevick approximation or the hypernetted-chain theory.
It can also be determined experimentally, by radiation scattering techniques or by direct visualization for large enough (micrometer-sized) particles via traditional or confocal microscopy.
The radial distribution function is of fundamental importance since it can be used, using the Kirkwood–Buff solution theory, to link the microscopic details to macroscopic properties.
Moreover, by the reversion of the Kirkwood–Buff theory, it is possible to attain the microscopic details of the radial distribution function from the macroscopic properties.
This is not true in general, and the order in which the positions occupy the argument slots of
The probability that those positions ARE occupied is found by summing over all configurations in which a particle is at each of those locations.
For fewer positions, we integrate over extraneous arguments, and include a correction factor to prevent overcounting,
, counting the number of ways one can sequentially pick particles to place at the
For a crystal it is a periodic function with sharp maxima at the lattice sites.
The partition function in this case is from which the definition gives the desired result In fact, for this special case every n-particle density is independent of coordinates, and can be computed explicitly
is of special importance, as it is directly related (via a Fourier transform) to the structure factor of the system and can thus be determined experimentally using X-ray diffraction or neutron diffraction.
the ensemble average, yielding: where the second equality requires the equivalence of particles
Since this contribution is inaccessible experimentally we can subtract it from the equation above and redefine the structure factor as a regular function: Finally, we rename
and the structure factor at the origin yields the compressibility equation: It can be shown[4] that the radial distribution function is related to the two-particle potential of mean force
, the average internal energy per particle is:[5]: Section 2.5 Developing the virial equation yields the pressure equation of state: The radial distribution function is an important measure because several key thermodynamic properties, such as potential energy and pressure can be calculated from it.
As the density of the gas increases, the low-density limit becomes less and less accurate since a particle situated in
[8] In recent years, some attention has been given to develop pair correlation functions for spatially-discrete data such as lattices or networks.
The technique can be used at very short length scales (down to the atomic level[10]) but involves significant space and time averaging (over the sample size and the acquisition time, respectively).
In this way, the radial distribution function has been determined for a wide variety of systems, ranging from liquid metals[11] to charged colloids.
directly by extracting particle positions from traditional or confocal microscopy.
[14] This technique is limited to particles large enough for optical detection (in the micrometer range), but it has the advantage of being time-resolved so that, aside from the statical information, it also gives access to dynamical parameters (e.g. diffusion constants[15]) and also space-resolved (to the level of the individual particle), allowing it to reveal the morphology and dynamics of local structures in colloidal crystals,[16] glasses,[17][18] gels,[19][20] and hydrodynamic interactions.
[21] Direct visualization of a full (distance-dependent and angle-dependent) pair correlation function was achieved by a scanning tunneling microscopy in the case of 2D molecular gases.
[22] It has been noted that radial distribution functions alone are insufficient to characterize structural information.
Distinct point processes may possess identical or practically indistinguishable radial distribution functions, known as the degeneracy problem.
[23][24] In such cases, higher order correlation functions are needed to further describe the structure.
were less studied, since they are generally less important for the thermodynamics of the system; at the same time, they are not accessible by conventional scattering techniques.
They can however be measured by coherent X-ray scattering and are interesting insofar as they can reveal local symmetries in disordered systems.