COVID-19
[62] Reducing the risk of long COVID includes staying up to date on the most recent COVID-19 vaccine, practicing good hygiene, maintaining clean indoor air, and physical distancing from people infected with a respiratory virus.
[91] Droplets that are below a certain critical size, generally thought to be <100μm diameter, evaporate faster than they settle; due to that fact, they form respiratory aerosol particles that remain airborne for a long period of time over extensive distances.
[111][112] The WHO, in collaboration with partners, expert networks, national authorities, institutions and researchers, have established nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages by GISAID, Nextstrain and Pango.
The expert group convened by the WHO recommended the labelling of variants using letters of the Greek alphabet, for example, Alpha, Beta, Delta, and Gamma, giving the justification that they "will be easier and more practical to discussed by non-scientific audiences".
[134] While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain.
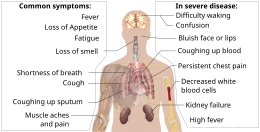
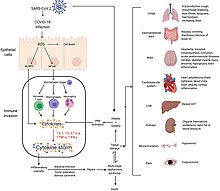
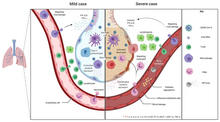
[159][needs update] Additionally, people with COVID‑19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.
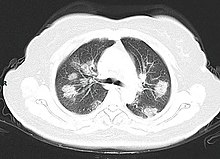
[175][189] Characteristic imaging features on chest radiographs and computed tomography (CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without pleural effusions.
[192] The main pathological findings at autopsy are: Preventive measures to reduce the chances of infection include getting vaccinated, staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, ventilating indoor spaces, managing potential exposure durations,[198] washing hands with soap and water often and for at least twenty seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands.
[233] Displacement ventilation with large natural inlets can move stale air directly to the exhaust in laminar flow while significantly reducing the concentration of droplets and particles.
[246] Evidence indicates that contact with infected surfaces is not the main driver of COVID‑19,[247][248][249] leading to recommendations for optimised disinfection procedures to avoid issues such as the increase of antimicrobial resistance through the use of inappropriate cleaning products and processes.
[254] On many surfaces, including glass, some types of plastic, stainless steel, and skin, the virus can remain infective for several days indoors at room temperature, or even about a week under ideal conditions.
In these, supportive care includes medication such as paracetamol or NSAIDs to relieve symptoms (fever, body aches, cough), proper intake of fluids, rest, and nasal breathing.
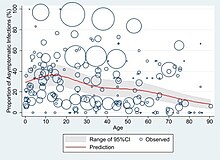
[334] Overall, approximately one-third of those investigated after four weeks will have findings of pulmonary fibrosis or reduced lung function as measured by DLCO, even in asymptomatic people, but with the suggestion of continuing improvement with the passing of more time.
[387][388][389] More severe impacts upon patients including the relative incidence of the necessity of hospitalisation requirements, and vulnerability to the disease has been associated via DNA analysis to be expressed in genetic variants at chromosomal region 3, features that are associated with European Neanderthal heritage.
[102][398][399] A joint-study conducted in early 2021 by the People's Republic of China and the World Health Organization indicated that the virus descended from a coronavirus that infects wild bats, and likely spread to humans through an intermediary wildlife host.
[401] According to articles published in July 2022 in Science, virus transmission into humans occurred through two spillover events in November 2019 and was likely due to live wildlife trade on the Huanan wet market in the city of Wuhan (Hubei, China).
[26][27] Available evidence suggests that the SARS-CoV-2 virus was originally harboured by bats, and spread to humans multiple times from infected wild animals at the Huanan Seafood Market in Wuhan in December 2019.
[434] Eight of these doctors, including Li Wenliang (punished on 3 January),[435] were later admonished by the police for spreading false rumours and another, Ai Fen, was reprimanded by her superiors for raising the alarm.
[443] A report in The Lancet on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential".
[453] A September 2020 review journal article said, "The possibility that the COVID‑19 infection had already spread to Europe at the end of last year is now indicated by abundant, even if partially circumstantial, evidence", including pneumonia case numbers and radiology in France and Italy in November and December.
[475] Modelling research has been conducted with several objectives, including predictions of the dynamics of transmission,[476] diagnosis and prognosis of infection,[477] estimation of the impact of interventions,[478][479] or allocation of resources.
[493] In June, initial results from the randomised RECOVERY Trial in the United Kingdom showed that dexamethasone reduced mortality by one third for people who are critically ill on ventilators and one fifth for those receiving supplemental oxygen.
[500][501] In September 2020, the European Medicines Agency (EMA) endorsed the use of dexamethasone in adults and adolescents from twelve years of age and weighing at least 40 kilograms (88 lb) who require supplemental oxygen therapy.
[504] Bamlanivimab is authorised for people with positive results of direct SARS-CoV-2 viral testing who are twelve years of age and older weighing at least 40 kilograms (88 lb), and who are at high risk for progressing to severe COVID‑19 or hospitalisation.
[504] In February 2021, the FDA issued an emergency use authorisation (EUA) for bamlanivimab and etesevimab administered together for the treatment of mild to moderate COVID‑19 in people twelve years of age or older weighing at least 40 kilograms (88 lb) who test positive for SARS‑CoV‑2 and who are at high risk for progressing to severe COVID‑19.
[505] In April 2021, the FDA revoked the emergency use authorisation (EUA) that allowed for the investigational monoclonal antibody therapy bamlanivimab, when administered alone, to be used for the treatment of mild-to-moderate COVID‑19 in adults and certain paediatric patients.
[513] The interleukin-6 receptor (IL-6R) antagonist was approved by the FDA to undergo a Phase III clinical trial assessing its effectiveness on COVID‑19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017.
[515] Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T cells in hospitalised patients with COVID‑19.
This involves the production of convalescent serum, which consists of the liquid portion of the blood from people who recovered from the infection and contains antibodies specific to this virus, which is then administered to active patients.
[517] An updated Cochrane review in May 2023 found high certainty evidence that, for the treatment of people with moderate to severe COVID‑19, convalescent plasma did not reduce mortality or bring about symptom improvement.