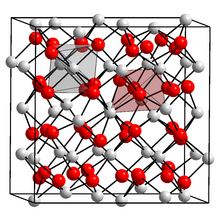
Corundum (structure)
Corundum is the name for a structure prototype in inorganic solids, derived from the namesake polymorph of aluminum oxide (α-Al2O3).
[1] Other compounds, especially among the inorganic solids, exist in corundum structure, either in ambient or other conditions.
Corundum structures are associated with metal-insulator transition,[2] ferroelectricity,[3] polar magnetism,[4][5] and magnetoelectric effects.
It typically exists in binary compounds of the type A2B3, where A is metallic and B is nonmetallic, including sesquioxides (A2O3),[6] sesquisulfides (A2S3),[7] etc.
The corundum-like structure with the composition A2BB'O6 is called double corundum.