Curium
Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley.
Curium is used in making heavier actinides and the 238Pu radionuclide for power sources in artificial cardiac pacemakers and RTGs for spacecraft.
It served as the α-source in the alpha particle X-ray spectrometers of several space probes, including the Sojourner, Spirit, Opportunity, and Curiosity Mars rovers and the Philae lander on comet 67P/Churyumov–Gerasimenko, to analyze the composition and structure of the surface.
Following cyclotron irradiation of the oxide, the coating was dissolved with nitric acid and then precipitated as the hydroxide using concentrated aqueous ammonia solution.
The separation of curium and americium was so painstaking that the Berkeley group initially called those elements pandemonium (from Greek for all demons or hell) and delirium (from Latin for madness).
Louis Werner and Isadore Perlman made the first substantial sample of 30 μg curium-242 hydroxide at University of California, Berkeley in 1947 by bombarding americium-241 with neutrons.
[17][18] A synthetic, radioactive element, curium is a hard, dense metal with a silvery-white appearance and physical and chemical properties resembling gadolinium.
At pressure >23 GPa, at room temperature, α-Cm becomes β-Cm, which has face-centered cubic symmetry, space group Fm3m and lattice constant a = 493 pm.
Curium's resistivity is similar to that of gadolinium, and the actinides plutonium and neptunium, but significantly higher than that of americium, uranium, polonium and thorium.
[4] Under ultraviolet illumination, curium(III) ions show strong and stable yellow-orange fluorescence with a maximum in the range of 590–640 nm depending on their environment.
Analysis of this fluorescence allows monitoring interactions between Cm(III) ions in organic and inorganic complexes.
[35] Curium in its complexes commonly exhibits a 9-fold coordination environment, with a tricapped trigonal prismatic molecular geometry.
Californium is a strong neutron emitter, and would pollute the back end of the fuel cycle and increase the dose to reactor personnel.
The longest-lived isotope, 247Cm, has half-life 15.6 million years; so any primordial curium, that is, present on Earth when it formed, should have decayed by now.
[53] Analysis of the debris at the test site of the United States' first thermonuclear weapon, Ivy Mike (1 November 1952, Enewetak Atoll), besides einsteinium, fermium, plutonium and americium also revealed isotopes of berkelium, californium and curium, in particular 245Cm, 246Cm and smaller quantities of 247Cm, 248Cm and 249Cm.
[57] Curium is made in small amounts in nuclear reactors, and by now only kilograms of 242Cm and 244Cm have been accumulated, and grams or even milligrams for heavier isotopes.
Further neutron capture followed by β−-decay gives americium (241Am) which further becomes 242Cm: For research purposes, curium is obtained by irradiating not uranium but plutonium, which is available in large amounts from spent nuclear fuel.
[61] Separation of curium from the very chemically similar americium can also be done by treating a slurry of their hydroxides in aqueous sodium bicarbonate with ozone at elevated temperature.
The reaction was done in an environment free of water and oxygen, in an apparatus made of tantalum and tungsten, using elemental barium or lithium as reducing agents.
[29][68] Upon heating to 600–650 °C in vacuum (about 0.01 Pa), it transforms into the whitish Cm2O3:[29][69] Or, Cm2O3 can be obtained by reducing CmO2 with molecular hydrogen:[70] Also, a number of ternary oxides of the type M(II)CmO3 are known, where M stands for a divalent metal, such as barium.
[25][84] Dissolved Cm3+ ions bind with many organic compounds, such as hydroxamic acid,[85] urea,[86] fluorescein[87] and adenosine triphosphate.
The resulting complexes show strong yellow-orange emission under UV light excitation, which is convenient not only for their detection, but also for studying interactions between the Cm3+ ion and the ligands via changes in the half-life (of the order ~0.1 ms) and spectrum of the fluorescence.
[26][85][86][87][88] There are a few reports on biosorption of Cm3+ by bacteria and archaea,[89][90] and in the laboratory both americium and curium were found to support the growth of methylotrophs.
Its two most common isotopes 242Cm and 244Cm are strong alpha emitters (energy 6 MeV); they have fairly short half-lives, 162.8 days and 18.1 years, and give as much as 120 W/g and 3 W/g of heat, respectively.
243Cm with a ~30-year half-life and good energy yield of ~1.6 W/g could be a suitable fuel, but it gives significant amounts of harmful gamma and beta rays from radioactive decay products.
[96] The odd-mass curium isotopes 243Cm, 245Cm, and 247Cm are all highly fissile and can release additional energy in a thermal spectrum nuclear reactor.
[55][99][100] An elaborate APXS setup has a sensor head containing six curium sources with a total decay rate of several tens of millicuries (roughly one gigabecquerel).
[53][55] Curium is absorbed in the body much more strongly via inhalation, and the allowed total dose of 244Cm in soluble form is 0.3 μCi.
[14] Intravenous injection of 242Cm- and 244Cm-containing solutions to rats increased the incidence of bone tumor, and inhalation promoted lung and liver cancer.
[103] Such a procedure involves several steps, where curium is first separated and then converted by neutron bombardment in special reactors to short-lived nuclides.





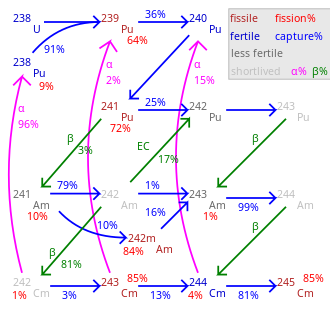
Fission percentage is 100 minus shown percentages.
Total rate of transmutation varies greatly by nuclide.
245 Cm– 248 Cm are long-lived with negligible decay.