Radium
In nearly all of its applications, radium has been replaced with less dangerous radioisotopes, with one of its few remaining non-medical uses being the production of actinium in nuclear reactors.
[4] Pure radium is a volatile, lustrous silvery-white metal, even though its lighter congeners calcium, strontium, and barium have a slight yellow tint.
[7] Like barium and the alkali metals, radium crystallizes in the body-centered cubic structure at standard temperature and pressure: the radium–radium bond distance is 514.8 picometers.
[8] Radium has a density of 5.5 g/cm3, higher than that of barium, and the two elements have similar crystal structures (bcc at standard temperature and pressure).
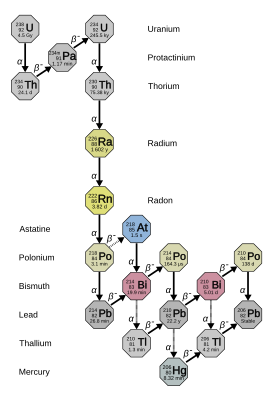
Its immediate decay product is the dense radioactive noble gas radon (specifically the isotope 222Rn), which is responsible for much of the danger of environmental radium.
[14][b] It is 2.7 million times more radioactive than the same molar amount of natural uranium (mostly uranium-238), due to its proportionally shorter half-life.
The ionizing radiation emitted by radium bromide excites nitrogen molecules in the air, making it glow.
[27] The large ionic radius of Ra2+ (148 pm) results in weak ability to form coordination complexes and poor extraction of radium from aqueous solutions when not at high pH.
Because of its relative longevity, 226Ra is the most common isotope of the element, making up about one part per trillion of the Earth's crust; essentially all natural radium is 226Ra.
[31] Radium was discovered by Marie Skłodowska-Curie and her husband Pierre Curie on 21 December 1898 in a uraninite (pitchblende) sample from Jáchymov.
[36] This amalgam was then heated in an atmosphere of hydrogen gas to remove the mercury, leaving pure radium metal.
[38][39] Radium metal was first industrially produced at the beginning of the 20th century by Biraco, a subsidiary company of Union Minière du Haut Katanga (UMHK) in its Olen plant in Belgium.
Additionally, for several years the companies had attempted to cover up the effects and avoid liability by insisting that the Radium Girls were instead suffering from syphilis.
[47] As a result of the lawsuit, and an extensive study by the U.S. Public Health Service, the adverse effects of radioactivity became widely known, and radium-dial painters were instructed in proper safety precautions and provided with protective gear.
[55] Radium was once an additive in products such as cosmetics, soap, razor blades, and even beverages due to its supposed curative powers.
[61] As of 2011, safer gamma emitters such as 60Co, which is less costly and available in larger quantities, were usually used to replace the historical use of radium in this application,[28] but factors including increasing costs of cobalt and risks of keeping radioactive sources on site have led to an increase in the use of linear particle accelerators for the same applications.
As early as 1904, Daniel MacDougal used radium in an attempt to determine whether it could provoke sudden large mutations and cause major evolutionary shifts.
Nobel-winning biologist Hermann Muller briefly studied the effects of radium on fruit fly mutations before turning to more affordable x-ray experiments.
This ionization ensures reliable and consistent operation by providing a steady current when a high voltage is applied, enhancing the device's performance and stability.
The first steps of the radium extraction process involved boiling with sodium hydroxide, followed by hydrochloric acid treatment to minimize impurities of other compounds.
[67] The formation of an Austrian monopoly and the strong urge of other countries to have access to radium led to a worldwide search for uranium ores.
[69] As of 1997 the chief radium-producing countries were Belgium, Canada, the Czech Republic, Slovakia, the United Kingdom, and Russia.
More recently discovered radioisotopes, such as cobalt-60 and caesium-137, are replacing radium in even these limited uses because several of these isotopes are more powerful emitters, safer to handle, and available in more concentrated form.
[78] The isotope 223Ra was approved by the United States Food and Drug Administration in 2013 for use in medicine as a cancer treatment of bone metastasis in the form of a solution[79] including radium-223 chloride.
[82] Radium was still used in 2007 as a radiation source in some industrial radiography devices to check for flawed metallic parts, similarly to X-ray imaging.
The French physicist Antoine Becquerel carried a small ampoule of radium in his waistcoat pocket for six hours and reported that his skin became ulcerated.
[92] The International Atomic Energy Agency (IAEA) publishes safety standards and provides recommendations for the handling of and exposure to radium in its works on naturally occurring radioactive materials and the broader International Basic Safety Standards,[93] which are not enforced by the IAEA but are available for adoption by members of the organization.
[94] In addition, in efforts to reduce the quantity of old radiotherapy devices that contain radium, the IAEA has worked since 2022[95] to manage and recycle disused 226Ra sources.
Radium sources themselves, rather than worker exposures, are regulated more closely by the Nuclear Regulatory Commission,[100] which requires licensing for anyone possessing 226Ra with activity of more than 0.01 μCi.
[101] The particular governing bodies that regulate radioactive materials and nuclear energy are documented by the Nuclear Energy Agency for member countries[102] – for instance, in the Republic of Korea, the nation's radiation safety standards are managed by the Korea Radioisotope Institute, established in 1985, and the Korea Institute of Nuclear Safety, established in 1990[103] – and the IAEA leads efforts in establishing governing bodies in locations that do not have government regulations on radioactive materials.


- Radium-226 radiation source.
- Activity 3300 Bq (3.3 kBq)