Cyanine
Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV.
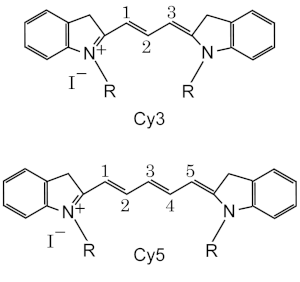
Cyanines have been classified in many ways:[3] Additionally, these classes are recognized:[4] where two quaternary nitrogens are joined by a polymethine chain.
[5] Both nitrogens may each be independently part of a heteroaromatic moiety, such as pyrrole, imidazole, thiazole, pyridine, quinoline, indole, benzothiazole, etc.
For applications to biotechnology, special cyanine dyes are synthesized from 2, 3, 5 or 7-methine structures with reactive groups on either one or both of the nitrogen ends so that they can be chemically linked to either nucleic acids or protein molecules.
Cyanine dyes are available with different modifications such as methyl, ethyl or butyl substituents, carboxyl, acetylmethoxy, and sulfo groups which alter their hydrophilicity.
[11] Because they yield brighter and more stable fluorescence, cyanines can advantageously replace conventional dyes such as fluorescein and rhodamines.
Due to its high molar extinction coefficient, this dye is also easily detected by naked eye on electrophoresis gels, and in solution.
Cy5 became a popular replacement for far red fluorescent dyes because of its high extinction coefficient (as small as 1 nanomol can be detected in gel electrophoresis by naked eye) and its fluorophore emission maximum in the red region, where many CCD detectors have maximum sensitivity and biological objects give low background interference.
Cyanine dyes are used to label proteins, antibodies, peptides, nucleic acid probes, and any kind of other biomolecules to be used in a variety of fluorescence detection techniques: flow cytometry, microscopy (mainly the visible range, but also UV and IR), microplate assays, microarrays, as well as "light-up probes," and in vivo imaging.
[18] In micorarray experiments DNA or RNA is labeled with either Cy3 or Cy5 that has been synthesized to carry an N-hydroxysuccinimidyl ester (NHS-ester) reactive group.
These variations make it extremely difficult, if not impossible, to use computers to automate the acquisition of the data after the separation is complete.

I = Streptocyanines,
II = Hemicyanines,
III = Closed cyanines