Quenching (fluorescence)
[8][9] Many dyes undergo self-quenching, which can decrease the brightness of protein-dye conjugates for fluorescence microscopy,[10] or can be harnessed in sensors of proteolysis.
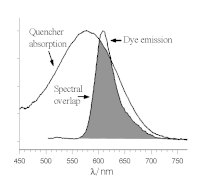
FRET is based on classical dipole-dipole interactions between the transition dipoles of the donor and acceptor and is extremely dependent on the donor-acceptor distance, R, falling off at a rate of 1/R6.
FRET also depends on the donor-acceptor spectral overlap (see figure) and the relative orientation of the donor and acceptor transition dipole moments.
[12] Dexter electron transfer is a short-range phenomenon that falls off exponentially with distance (proportional to e−kR where k is a constant that is the inverse of the sum of both van der Waals radius of the atom over 2 [13]) and depends on spatial overlap of donor and quencher molecular orbitals.
Collisional quenching occurs when the excited fluorophore experiences contact with an atom or molecule that can facilitate non-radiative transitions to the ground state.