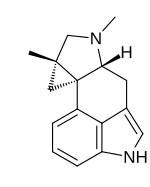
Cycloclavine
[6] In 2016, Wipf and McCabe completed an 8-step asymmetric synthesis of (–)-cycloclavine,[7] and in 2018, they expanded this approach toward (+)-cycloclavine and a biological characterization of the binding profile of both enantiomers on 16 brain receptors.
[8] Natural (+)- and unnatural (–)-cycloclavine demonstrated significant stereospecificity and unique binding profiles in comparison to LSD (lysergic acid diethylamide), psilocin, and DMT.
Differential 5-HT receptor affinities, as well as novel sigma-1 receptor properties, suggest potential future therapeutic opportunities of clavine alkaloid scaffolds.
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.
(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui)