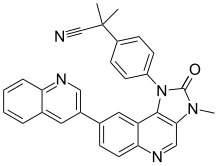
Dactolisib
[5] A phase IB/II clinical trial for locally advanced or metastatic HER2 negative breast cancer has completed.
[6] A phase II clinical trial for advanced pancreatic neuroendocrine tumors (pNET) had initially reported results, but was later terminated because insufficient normal tissue tolerance to the drug.
[8] Another Phase Ib study on patients with various solid cancers found severe normal tissue toxicity as well when BEZ235/Dactolisib was administered in combination with the mTOR inhibitor Everolimus.
BEZ235 systemic exposure increased in a dose-proportional manner while oral bioavailability was quite low, which may be related to gastrointestinal-specific toxicity .
[10] A phase 2a randomized, placebo-controlled clinical trial published in 2018 showed that everolimus in combination with dactolisib decreased the rate of reported infections in an elderly population.