David M. Lemal
[5] Lemal began his independent academic career at the University of Wisconsin in Madison, first as instructor (1958–60), and then as assistant professor (1960–65).
At Wisconsin, Lemal developed a career-long interest in highly strained molecules, short-lived species and concerted reactions.
During his career, Lemal mentored in research more than a hundred undergraduates, graduate students, and postdoctoral fellows, and taught chemistry courses for 50 years.
[9] These strained molecules were remarkably robust and thermally stable as compared with their rather fragile hydrocarbon counterparts, and that intriguing contrast led to further exploration of fluorocarbon chemistry.
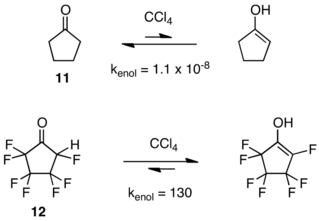
[12] The effect has proved to be general, but if fluoro instead of perfluoroalkyl groups decorate the carbon skeleton, behavior can be very different, as the following example reveals.
Another case of contrasting chemistry emerged when Lemal’s group prepared perfluoroquadricyclane (6) by a photoreaction at -30 °C and allowed it to warm above 0 °C.
[20] In 1966 Lemal and coworkers studied a class of reactions that would come to be called cheletropic, cyclic concerted processes in which two bonds are formed or broken at the same atom.
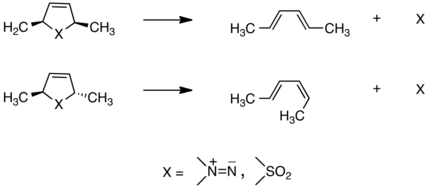
Finding that they take place stereospecifically, the findings built on ideas of Woodward and Hoffmann to develop independently a theory of this class of transformations that explained the group's experimental results, shown here:[21][22] A decade later, when it was generally accepted that all cyclic, concerted processes (termed pericyclic) obey the Woodward-Hoffmann orbital symmetry rules (of which those for cheletropic reactions are a subset), Lemal and co-workers discovered an extraordinarily facile degenerate rearrangement that led them to challenge that belief.
[26] In a long and continuing investigation that has combined experiment and computation to great effect, his group and later others have established that there are pseudopericyclic examples to be found in all of the classes of cyclic concerted processes.
In 1960, Wanzlick reported that tetraaminoethylene 1 readily dissociates into diaminocarbene halves,[27] and a long series of papers from his group followed, describing interesting reactions of 1 interpreted as arising from the carbene.
[31] By selecting a diaminocarbene structure intermediate in character between the Wanzlick and Arduengo carbenes, Lemal’s group found and studied a carbene-dimer pair that does exist in equilibrium.
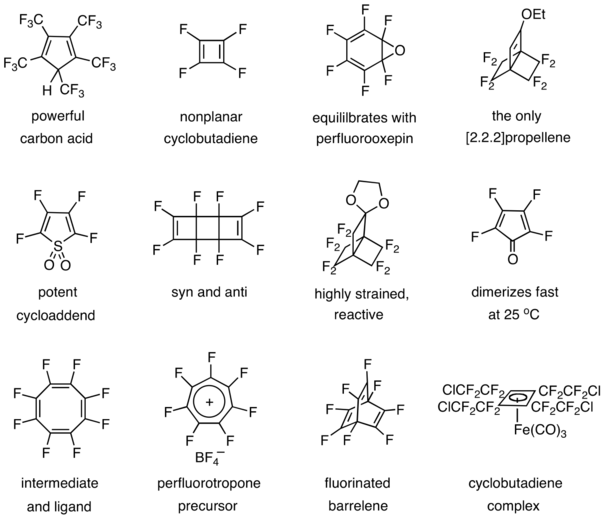
Other contributions include the preparation and studies of a powerful carbon acid,[33] tetrafluorocyclobutadiene,[34][35] hexafluorobenzene oxide,[36][37] a [2.2.2]propellene,[38] tetrafluorothiophene dioxide,[39] octafluorotricyclooctadiene,[40] a highly reactive propellane,[41] tetrafluorocyclopentadienone,[42] octafluorocyclooctatetraene,[43] a heptafluorotropylium salt, octafluorobarrelene ,[44] and a cyclobutadiene complex.