Death effector domain
The subfamilies resemble structurally one another, all of them (and DED in particular) are composed of a bundle of 6 alpha-helices, but they diverge in the surface features.
[3] DEDs in this protein show an asymmetric unit dimer, with its interface contains two hydrogen bonding networks, that appear as a filamentous structure.
As far as it is known, the homotypic interactions that activate caspase and trigger apoptosis are mediated by asymmetrical surface contacts between partners (like DED1 and DED2 in the caspase-8 case).
To know the role of DEDs in this process is important to observe the formation of the multiprotein death-including signalling complex (DISC).
Each interaction involves an area of 1062 Å2 and contributions from hydrophobic side chains, hydrogen bonding and salt bridges.
FLIPL’s pseudo-caspase has two tandem DEDs that are very similar to the N-terminus of capase-8, but in which there is an important mutation in the active site (cysteine to tyrosine).
This heterodimeration done between their DEDs prevents from the normal homodimeration so that the pseudo-caspase is unable to activate the apoptotic cascade.
In this case heterodimerisation directly fails to activate procaspase-8 as the initial conformational change cannot take place in procaspase-8’s caspase domain.
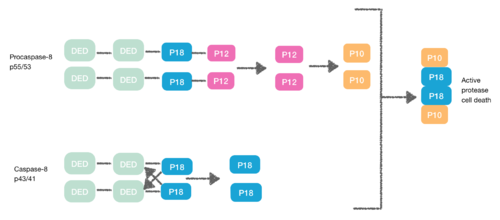
Structure: There are two groups of proteases: The two tandem DEDs in the pro-domain of caspase induce the protein-protein interactions with other proteins like the FADD.
PED is a small, non-catalytic, protein consisting of an N-terminal death-effector domain (DED) and a C-terminal tail with irregular structure.
[10] Besides apoptosis, PEA-15 inhibits the insulin-mediated glucose transport in muscle cells, so a high level expression of PEA-15's mRNA has been associated to diabetes mellitus type II.
Shows DNA binding capacity, localized in the nucleoli in overexpression where it associates with a molecule called DEDAF (DED-associated factor) that potentiates apoptosis.
It is noted to interact with c-FLIP and DEDD and to have an important role in polymerase II-dependent transcription repression.
[14] HIP-1 contains a pseudo death effector domain (pDED), that's why the overexpression of HIP-1 induces apoptosis in several cells as DED proteins do.
[7] DED complexes have been shown to function at crucial steps controlling life and death cell processes.
However, the abnormal apoptosis it is not exclusive from cancer, there are other pathologies such as inflammation and neurodegenerative diseases than can also be treated with these kind of therapeutics.