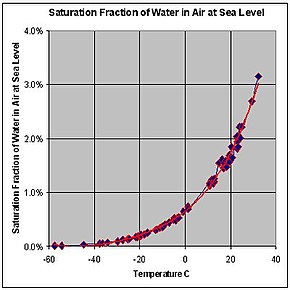
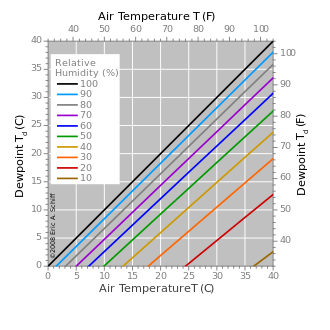
Dew point
The dew point is the temperature the air needs to be cooled to (at constant pressure) in order to achieve a relative humidity of 100%[1].
[6]: 129 In technical terms, the dew point is the temperature at which the water vapor in a sample of air at constant barometric pressure condenses into liquid water at the same rate at which it evaporates.
[7] At temperatures below the dew point, the rate of condensation will be greater than that of evaporation, forming more liquid water.
The condensed water is called dew when it forms on a solid surface, or frost if it freezes.
In the air, the condensed water is called either fog or a cloud, depending on its altitude when it forms.
[9] A high relative humidity implies that the dew point is close to the current air temperature.
A relative humidity of 100% indicates the dew point is equal to the current temperature and that the air is maximally saturated with water.
[10] General aviation pilots use dew point data to calculate the likelihood of carburetor icing and fog, and to estimate the height of a cumuliform cloud base.
[11] This means that, if the pressure increases, the mass of water vapor per volume unit of air must be reduced in order to maintain the same dew point.
Because Denver is at a higher elevation than New York, it will tend to have a lower barometric pressure.
This means that if the dew point and temperature in both cities are the same, the amount of water vapor in the air will be greater in Denver.
A wet bulb thermometer also uses evaporative cooling, so it provides a good measure for use in evaluating comfort level.
[citation needed] The drier air can cause skin to crack and become irritated more easily.
The US Occupational Safety and Health Administration recommends indoor air be maintained at 20–24.5 °C (68–76 °F) with a 20–60% relative humidity,[13] equivalent to a dew point of approximately 4.0 to 16.5 °C (39 to 62 °F) (by Simple Rule calculation below).
People inhabiting tropical and subtropical climates acclimatize somewhat to higher dew points.
[14] Devices called hygrometers are used to measure dew point over a wide range of temperatures.
These devices consist of a polished metal mirror which is cooled as air is passed over it.
The dew point is revealed by observing the loss of clarity in the reflection cast by the mirror.
Manual devices of this sort can be used to calibrate other types of humidity sensors, and automatic sensors may be used in a control loop with a humidifier or dehumidifier to control the dew point of the air in a building or in a smaller space for a manufacturing process.
A well-known empirical approximation used to calculate the dew point, Td, given just the actual ("dry bulb") air temperature, T (in degrees Celsius) and relative humidity (in percent), RH, is the Magnus formula:
The more complete formulation and origin of this approximation involves the interrelated saturated water vapor pressure (in units of millibars, also called hectopascals) at T, Ps(T), and the actual vapor pressure (also in units of millibars), Pa(T), which can be either found with RH or approximated with the barometric pressure (in millibars), BPmbar, and "wet-bulb" temperature, Tw is (unless declared otherwise, all temperatures are expressed in degrees Celsius):
For greater accuracy, Ps(T) (and therefore γ(T, RH)) can be enhanced, using part of the Bögel modification, also known as the Arden Buck equation, which adds a fourth constant d:
The ones used in NOAA's presentation[18] are taken from a 1980 paper by David Bolton in the Monthly Weather Review:[19] These valuations provide a maximum error of 0.1%, for −30 °C ≤ T ≤ 35°C and 1% < RH < 100%.
Also noteworthy is the Sonntag1990,[20] Another common set of values originates from the 1974 Psychrometry and Psychrometric Charts.
Two particular sets provide a range of −40 °C to +50 °C between the two, with even lower maximum error within the indicated range than all the sets above: There is also a very simple approximation that allows conversion between the dew point, temperature, and relative humidity.
This can be expressed as a simple rule of thumb: For every 1 °C difference in the dew point and dry bulb temperatures, the relative humidity decreases by 5%, starting with RH = 100% when the dew point equals the dry bulb temperature.
The derivation of this approach, a discussion of its accuracy, comparisons to other approximations, and more information on the history and applications of the dew point, can be found in an article published in the Bulletin of the American Meteorological Society.
For example, a relative humidity of 100% means dew point is the same as air temp.
The frost point is similar to the dew point in that it is the temperature to which a given parcel of humid air must be cooled, at constant atmospheric pressure, for water vapor to be deposited on a surface as ice crystals without undergoing the liquid phase (compare with sublimation).
The frost point for a given parcel of air is always higher than the dew point, as breaking the stronger bonding between water molecules on the surface of ice compared to the surface of (supercooled) liquid water requires a higher temperature.