Dextromethorphan
[6] In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropion to serve as a rapid-acting antidepressant in people with major depressive disorder.
[11][12] Dextromethorphan and its major metabolite, dextrorphan, also block the NMDA receptor at high doses, which produces effects similar to other dissociative anesthetics such as ketamine, nitrous oxide, and phencyclidine.
[14][15] In 2022, the combination with brompheniramine and pseudoephedrine was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions.
In 2010, the FDA approved the combination drug dextromethorphan/quinidine under the brand name Nuedexta[19] for the treatment of pseudobulbar affect (uncontrollable laughing/crying).
[18] Dextromethorphan was once thought to cause Olney's lesions when administered intravenously; however, this was later proven inconclusive, due to lack of research on humans.
Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA receptor antagonists such as PCP, but not with dextromethorphan.
[5] Since dextromethorphan also acts as a serotonin reuptake inhibitor, users report that regular recreational use over a long period of time can cause withdrawal symptoms similar to those of antidepressant discontinuation syndrome.
Combining alcohol with dextromethorphan significantly increases the risk of overdose and other severe health complications, according to the NIAAA.
[38] Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the blood–brain barrier.
Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme CYP2D6.
[citation needed] Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.
Some types of medications known to inhibit CYP2D6 include certain SSRIs and tricyclic antidepressants, some antipsychotics, and the commonly available antihistamine diphenhydramine.
Even though many of the syntheses have been known since the middle of the 20th century, researchers are still working today to further develop the synthesis of Dextromethorphan and, for example, to make it more environmentally friendly.
[45] Formylation of octabase prior to cyclization avoids ether cleavage as a side reaction and yields higher than without N-substitution or N-methylation.
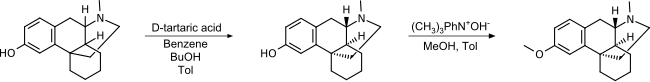
A resolution of the two isomers of racemorphan with tartaric acid was published in 1952,[46] and dextromethorphan was successfully tested in 1954 as part of US Navy and CIA-funded research on nonaddictive substitutes for codeine.
[48] The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about dextromethorphan, and online discussion groups formed around use and acquisition of the drug.
Indonesia is the only country that makes single-component dextromethorphan illegal over the counter and by prescription[51] and violators may be prosecuted by law.
National Anti-Narcotics Agency (BNN RI) has threatened to revoke pharmacies' and drug stores' licenses if they still stock dextromethorphan, and will notify the police for criminal prosecution.
[48] At doses much higher than medically recommended, dextromethorphan and its major metabolite, dextrorphan, acts as an NMDA receptor antagonist, which produces dissociative hallucinogenic states somewhat similar to ketamine and phencyclidine.
The second plateau is likened to a state of being on moderate amounts of alcohol and cannabis at the same time, featuring euphoria, sedation and minor hallucinations.
Reaching the fourth plateau is said to cause extreme sedation and a significant hallucinatory state as well as complete dissociation from reality.
[57] The combination drug dextromethorphan/quinidine (AVP-923),[58][59] traditionally used to treat pseudobulbar affect, is under investigation for the treatment of a variety of other neurological and neuropsychiatric conditions including agitation associated with Alzheimer's disease, among others.
[20][60] In 2013, a randomized clinical trial found that dextromethorphan may reduce the overall discomfort and duration of withdrawal symptoms associated with opioid use disorder.