Diamond cubic
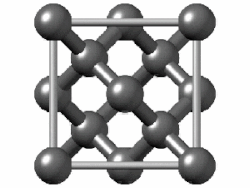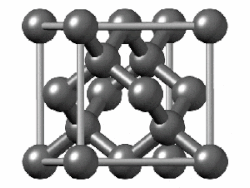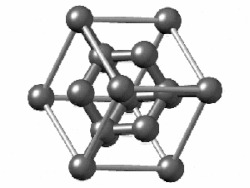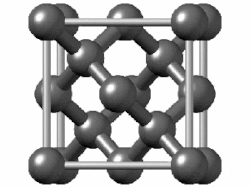
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify.
While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the semiconductors silicon and germanium, and silicon–germanium alloys in any proportion.
The lattice describes the repeat pattern; for diamond cubic crystals this lattice is "decorated" with a motif of two tetrahedrally bonded atoms in each primitive cell, separated by 1/4 of the width of the unit cell in each dimension.
Many compound semiconductors such as gallium arsenide, β-silicon carbide, and indium antimonide adopt the analogous zincblende structure, where each atom has nearest neighbors of an unlike element.
[3] significantly smaller (indicating a less dense structure) than the packing factors for the face-centered and body-centered cubic lattices.
[4] Zincblende structures have higher packing factors than 0.34 depending on the relative sizes of their two component atoms.
The first-, second-, third-, fourth-, and fifth-nearest-neighbor distances in units of the cubic lattice constant are
Alternatively, each point of the diamond cubic structure may be given by four-dimensional integer coordinates whose sum is either zero or one.
The total difference in coordinate values between any two points (their four-dimensional Manhattan distance) gives the number of edges in the shortest path between them in the diamond structure.
Because the diamond structure forms a distance-preserving subset of the four-dimensional integer lattice, it is a partial cube.
[6] Yet another coordinatization of the diamond cubic involves the removal of some of the edges from a three-dimensional grid graph.
Moreover, the diamond crystal as a network in space has a strong isotropic property.
Similarly, truss systems that follow the diamond cubic geometry have a high capacity to withstand compression, by minimizing the unbraced length of individual struts.
[11] The diamond cubic geometry has also been considered for the purpose of providing structural rigidity[12][13] though structures composed of skeletal triangles, such as the octet truss, have been found to be more effective for this purpose.