Atomic packing factor
For one-component crystals (those that contain only one type of particle), the packing fraction is represented mathematically by where Nparticle is the number of particles in the unit cell, Vparticle is the volume of each particle, and Vunit cell is the volume occupied by the unit cell.
For multiple-component structures (such as with interstitial alloys), the APF can exceed 0.74.
For example, metals with a high atomic packing factor will have a higher "workability" (malleability or ductility), similar to how a road is smoother when the stones are closer together, allowing metal atoms to slide past one another more easily.
The majority of metals take on either the HCP, FCC, or BCC structure.
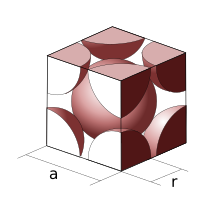
[2] For a simple cubic packing, the number of atoms per unit cell is one.
The side of the unit cell is of length 2r, where r is the radius of the atom.
For a face-centered cubic unit cell, the number of atoms is four.
Using geometry, and the side length, a can be related to r as: Knowing this and the formula for the volume of a sphere, it becomes possible to calculate the APF as follows: The primitive unit cell for the body-centered cubic crystal structure contains several fractions taken from nine atoms (if the particles in the crystal are atoms): one on each corner of the cube and one atom in the center.
Because the volume of each of the eight corner atoms is shared between eight adjacent cells, each BCC cell contains the equivalent volume of two atoms (one central and one on the corner).
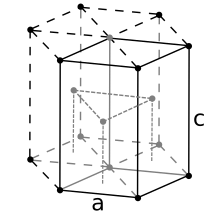
Therefore, the length of each side of the BCC structure can be related to the radius of the atom by Knowing this and the formula for the volume of a sphere, it becomes possible to calculate the APF as follows: For the hexagonal close-packed structure the derivation is similar.
Here the unit cell (equivalent to 3 primitive unit cells) is a hexagonal prism containing six atoms (if the particles in the crystal are atoms).
Indeed, three are the atoms in the middle layer (inside the prism); in addition, for the top and bottom layers (on the bases of the prism), the central atom is shared with the adjacent cell, and each of the six atoms at the vertices is shared with other six adjacent cells.