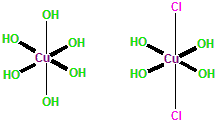
Dicopper chloride trihydroxide
[6] In neither of those applications was the polymorphic nature of the compound, or the size of individual particles of particular importance, so the manufacturing processes were simple precipitation schemes.
[6] Astable, free-flowing, non-dusty green powder with typical particle size of 30 ~ 100 microns has been used in preparation of uniform animal feed mixtures.
In the acidic pathway, the cupric chloride solution can be neutralized with caustic soda, or ammonia, lime, or other base.
Product with good particle size is produced and can be easily separated from background salt and other impurities in the mother liquor.
After simple rinse with water and drying, pure, free-flowing, non-dusty green crystalline solid with typical particle size of 30 ~ 100 micron is obtained.
Careful control of process conditions to favor the alpha polymorphs results in a product that remains free flowing over extended storage times, thus avoiding caking as occurs with both copper sulfate and the botallackite crystal form - also called beta basic copper chloride.
This process is used to manufacture thousands of tons of tribasic copper chloride every year, and has been the predominant route of commercial production since it was introduced by Steward in 1994.
[10] Atacamite and paratacamite crystal forms of Cu2(OH)3Cl have been found to be active species in supported CuCl2 catalyst systems for the oxidative carbonylation of methanol to dimethyl carbonate.
[13] A mixture of ultrafine powder CuO/Cu2(OH)3Cl has been shown to be good in photo-catalytic decolorization of dyes, such as amido black, and indigo carmine.
[14] Copper is one of the most critically important of the trace minerals that are essential elements in numerous enzymes that support metabolic functions in most organisms.
Since the early 1900s, copper has routinely been added to animal feedstuffs to support good health and normal development.
Starting in the 1950s, there was increasing focus on the issue of bioavailability of trace mineral supplements which led to copper sulfate pentahydrate becoming the predominant source.
Recognition that basic copper chloride would reduce feed stability problems led to issuance of patents on the use of the compound as a nutritional source.
Subsequently, animal feeding studies revealed that the alpha crystal form of basic copper chloride has a rate of chemical reactivity that is well matched to biological processes.
This form of the compound has proven to be particularly suitable as a commercial feed supplement for use in livestock and aquaculture due to its inherent chemical and physical characteristics.
It has been widely used in feed formulations for most species, including chickens, turkeys, pigs, beef and dairy cattle, horses, pets, aquaculture and exotic zoo animals.
It is the sugar-like coating of dark green glistening crystals found on many bronze objects from Egypt and Mesopotamia.
The resulting weak bonding between the sheets accounts for the perfect (100) cleavage and the typical platy habit of botallackite (Figure 2).
The existence of the regular octahedral [Cu(OH)6] is unusual, and it has been shown that partial substitution of Zn or Ni for Copper at this special site (3b) is necessary to stabilize paratacamite structure at ambient temperature.
Thermodynamic data based on the free energy of formation indicates that the order of stability of these polymorphs is clinoatacamite>atacamite> botallackite.
Spectroscopic studies show that the strength of hydrogen bonding in these polymorphs is in the order paratacamite >atacamite> botallackite.