Copper
Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement.
[10] Commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase.
[13] Copper, silver, and gold are in group 11 of the periodic table; these three metals have one s-orbital electron on top of a filled d-electron shell and are characterized by high ductility, and electrical and thermal conductivity.
[14] At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness.
This is due to the low plasma frequency of the metal, which lies in the red part of the visible spectrum, causing it to absorb the higher-frequency green and blue colors.
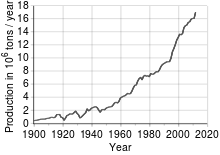
According to the British Geological Survey, in 2005, Chile was the top producer of copper with at least one-third of the world share followed by the United States, Indonesia and Peru.
[32] The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.
[33] An alternative source of copper for collection currently being researched are polymetallic nodules, which are located at the depths of the Pacific Ocean approximately 3000–6500 meters below sea level.
[52] Greenhouse gas emissions primarily arise from electricity consumed by the company, especially when sourced from fossil fuels, and from engines required for copper extraction and refinement.
Copper is one of the most important constituents of silver and karat gold solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.
[60] The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries.
[28] Shakudō is a Japanese decorative alloy of copper containing a low percentage of gold, typically 4–10%, that can be patinated to a dark blue or black color.
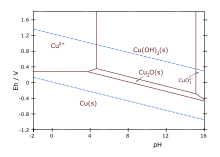
Specifically, using Benedict's reagent and Fehling's solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide.
[104] Natural bronze, a type of copper made from ores rich in silicon, arsenic, and (rarely) tin, came into general use in the Balkans around 5500 BC.
The rich copper deposits of Cornwall seem to have been largely untouched, in spite of extensive tin mining in the region, for reasons likely social and political rather than technological.
[121] The German scientist Gottfried Osann invented powder metallurgy in 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones.
Introduction of open pit steam shovel mining and innovations in smelting, refining, flotation concentration and other processing steps led to mass production.
[123] Flash smelting was developed by Outokumpu in Finland and first applied at Harjavalta in 1949; the energy-efficient process accounts for 50% of the world's primary copper production.
[133] Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its high electrical conductivity, tensile strength, ductility, creep (deformation) resistance, corrosion resistance, low thermal expansion, high thermal conductivity, ease of soldering, malleability, and ease of installation.
[141][142] The rapid growth of these sources in the 21st century has been prompted by increasing costs of fossil fuels as well as their environmental impact issues that significantly lowered their use.
When planning for a new renewable power facility, engineers and product specifiers seek to avoid supply shortages of selected materials.
[155][156][157][158] Roofs, flashings, rain gutters, downspouts, domes, spires, vaults, and doors have been made from copper for hundreds or thousands of years.
Copper's architectural use has been expanded in modern times to include interior and exterior wall cladding, building expansion joints, radio frequency shielding, and antimicrobial and decorative indoor products such as attractive handrails, bathroom fixtures, and counter tops.
Some of copper's other important benefits as an architectural material include low thermal movement, light weight, lightning protection, and recyclability.
In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet hardware, computer keyboards, health club equipment, and shopping cart handles.
[184] Copper may be used as a speculative investment due to the predicted increase in use from worldwide infrastructure growth, and the important role it has in producing wind turbines, solar panels, and other renewable energy sources.
[206][207] The U.S. Institute of Medicine (IOM) updated the estimated average requirements (EARs) and recommended dietary allowances (RDAs) for copper in 2001.
[220][221] In the US, the Occupational Safety and Health Administration (OSHA) has designated a permissible exposure limit (PEL) for copper dust and fumes in the workplace as a time-weighted average (TWA) of 1 mg/m3.
[222] The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3, time-weighted average.