Dihydrotestosterone
Dihydrotestosterone (DHT, 5α-dihydrotestosterone, 5α-DHT, androstanolone or stanolone) is an endogenous androgen sex steroid and hormone primarily involved in the growth and repair of the prostate and the penis, as well as the production of sebum and body hair composition.
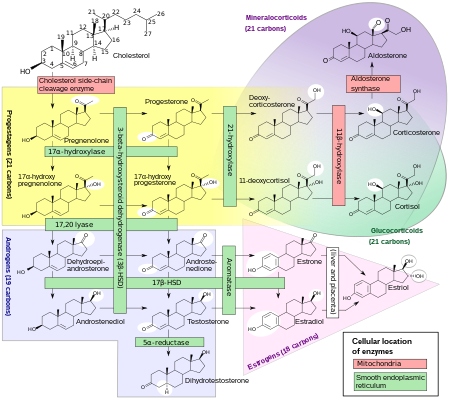
The enzyme 5α-reductase catalyzes the formation of DHT from testosterone in certain tissues including the prostate gland, seminal vesicles, epididymides, skin, hair follicles, liver, and brain.
DHT is biologically important for sexual differentiation of the male genitalia during embryogenesis, maturation of the penis and scrotum at puberty, growth of facial, body, and pubic hair, and development and maintenance of the prostate gland and seminal vesicles.
It is produced from the less potent testosterone by the enzyme 5α-reductase in select tissues, and is the primary androgen in the genitals, prostate gland, seminal vesicles, skin, and hair follicles.
[2] DHT signals act mainly in an intracrine and paracrine manner in the tissues in which it is produced, playing only a minor role, if any, as a circulating endocrine hormone.
Although castration results in 90-95% decrease of serum testosterone, DHT in the prostate is only decreased by 50%, supporting the notion that the prostate expresses necessary enzymes (including 5α-reductase) to produce DHT without testicular testosterone,[17] that outline the importance of 5α-reductase inhibitors.
[19][20] Much of the biological role of DHT has been elucidated in studies of individuals with congenital 5α-reductase type 2 deficiency, an intersex condition caused by a loss-of-function mutation in the gene encoding 5α-reductase type 2, the major enzyme responsible for the production of DHT in the body.
[13][21][2] It is characterized by a defective and non-functional 5α-reductase type 2 enzyme and a partial but majority loss of DHT production in the body.
[13] Genetic males (46,XY) with 5α-reductase type 2 deficiency are born with undervirilization including pseudohermaphroditism (ambiguous genitalia), pseudovaginal perineoscrotal hypospadias, and usually undescended testes.
Their external genitalia are female-like, with micropenis (a small, clitoris-like phallus), a partially unfused, labia-like scrotum, and a blind-ending, shallow vaginal pouch.
[13][24] Nonetheless, males with 5α-reductase type 2 deficiency exhibit signs of continued undervirilization in a number of domains.
Facial hair was absent or sparse in a relatively large group of Dominican males with the condition, known as the Güevedoces.
No temporal recession of the hairline or androgenic alopecia (pattern hair loss or baldness) has been observed in any of the cases of 5α-reductase type 2 deficiency that have been reported, whereas this is normally seen to some degree in almost all Caucasian males in their teenage years.
[12] In genetic males with 5α-reductase type 2 deficiency, the prostate gland is rudimentary or absent, and if present, remains small, underdeveloped, and unpalpable throughout life.
[28][29][30][22] As such, similarly to the case of 5α-reductase type 2 deficiency, they provide useful insights in the elucidation of the biological functions of DHT.
[15] In addition to prostate diseases, 5α-reductase inhibitors have subsequently been developed and introduced for the treatment of pattern hair loss in men.
[43] Conversely, it was found to decrease sebum DHT levels by 55% in men versus a modest reduction of only 15% for finasteride.
[8][13] This occurs in various tissues including the genitals (penis, scrotum, clitoris, labia majora),[55] prostate gland, skin, hair follicles, liver, and brain.
[46] SRD5A2 is most highly expressed in the genitals, prostate gland, epididymides, seminal vesicles, genital skin, facial and chest hair follicles,[58][59] and liver, while lower expression is observed in certain brain areas, non-genital skin/hair follicles, testes, and kidneys.
SRD5A1 is most highly expressed in non-genital skin/hair follicles, the liver, and certain brain areas, while lower levels are present in the prostate, epididymides, seminal vesicles, genital skin, testes, adrenal glands, and kidneys.
[8][70] These metabolites are in turn converted, respectively, into androsterone and epiandrosterone, then conjugated (via glucuronidation and/or sulfation), released into circulation, and excreted in urine.
[72][8] Ranges for circulating total DHT levels tested with HPLC–MS/MS and reported by LabCorp are as follows:[73] Ranges for circulating free DHT levels tested with HPLC–MS/MS and equilibrium dialysis and reported by LabCorp are as follows:[73] Other studies and labs assessing circulating total DHT levels with LC–MS/MS have reported ranges of 11–95 ng/dL (0.38–3.27 nmol/L) in adult men, 14–77 ng/dL (0.47–2.65 nmol/L) for healthy adult men (age 18–59 years), 23–102 ng/dL (0.8–3.5 nmol/L) for community-dwelling adult men (age <65 years), and 14–92 ng/dL (0.49–3.2 nmol/L) for healthy older men (age 71–87 years).
[5] There was no variation in DHT levels across the menstrual cycle in premenopausal women, which is in contrast to testosterone (which shows a peak at mid-cycle).
one of the original "underground" methods used to falsify drug testing in sport, as DHT does not alter the ratio of testosterone to epistestosterone in an athlete's urinary steroid profile, a measurement that was once the basis of drug tests used to detect steroid use.
[96] In 2004, Richard Auchus, in a review published in Trends in Endocrinology and Metabolism coined the term "backdoor pathway" as a metabolic route to DHT that: 1) bypasses conventional intermediates androstenedione and testosterone; 2) involves 5α-reduction of 21-carbon (C21) pregnanes to 19-carbon (C19) androstanes; and 3) involves the 3α-oxidation of 5α-androstane-3α,17β-diol to DHT.
[64] This review was based on earlier works (published in 2000–2004) by Shaw et al., Wilson et al., and Mahendroo et al., who studied DHT biosynthesis in tammar wallaby pouch young and mice.
[17] In 2011, Chang et al.[97] demonstrated that yet another metabolic pathway to DHT was dominant and possibly essential in castration-resistant prostate cancer (CRPC).