Dimethyl sulfoxide
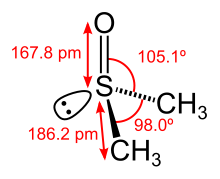
It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds,[6] with a nonbonded electron pair on the approximately tetrahedral sulfur atom.
[7] Its modern use as an industrial solvent began through popularization by Thor Smedslund at the Stepan Chemical Company.
The relative donor strength of DMSO toward a series of acids, versus other Lewis bases, can be illustrated by C-B plots.
[16][17] DMSO is a polar aprotic solvent and is less toxic than other members of this class, such as dimethylformamide, dimethylacetamide, N-methyl-2-pyrrolidone, and hexamethylphosphoramide (HMPA).
Because DMSO is only weakly acidic, it tolerates relatively strong bases and as such has been extensively used in the study of carbanions.
A set of non-aqueous pKa values (C-H, O-H, S-H and N-H acidities) for thousands of organic compounds have been determined in DMSO solution.
It has, for example, been employed as a co-solvent to assist absorption of the flavonol glycoside Icariin in the nematode worm Caenorhabditis elegans.
[23] For example, even a very low dose of DMSO has a powerful protective effect against paracetamol (acetaminophen)-induced liver injury in mice.
[26] DMSO in a PCR is applicable for supercoiled plasmids (to relax before amplification) or DNA templates with high GC-content (to decrease thermostability).
[citation needed] Use of DMSO in medicine dates from around 1963, when an Oregon Health & Science University Medical School team, headed by Stanley Jacob, discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system.
[30] Because DMSO increases the rate of absorption of some compounds through biological tissues, including skin, it is used in some transdermal drug delivery systems.
[31] DMSO has been examined for the treatment of numerous conditions and ailments, but the U.S. Food and Drug Administration (FDA) has approved its use only for the symptomatic relief of patients with interstitial cystitis.
As part of an autologous bone marrow transplant the DMSO is re-infused along with the patient's own hematopoietic stem cells.
[36] The use of DMSO as an alternative treatment for cancer is of particular concern, as it has been shown to interfere with a variety of chemotherapy drugs, including cisplatin, carboplatin, and oxaliplatin.
[34] One such distributor is Mildred Miller, who promoted DMSO for a variety of disorders and was consequently convicted of Medicare fraud.
[citation needed] The perceived garlic taste upon skin contact with DMSO may be due to nonolfactory activation of TRPA1 receptors in trigeminal ganglia.
DMSO is thought to increase the effects of blood thinners, steroids, heart medicines, sedatives, and other drugs.
[45] Nitrile gloves, which are very commonly used in chemical laboratories, may protect from brief contact but have been found to degrade rapidly with exposure to DMSO.
[47] Early clinical trials with DMSO were stopped because of questions about its safety, especially its ability to harm the eye.
[full citation needed] On September 9, 1965, The Wall Street Journal reported that a manufacturer of the chemical warned that the death of an Irish woman after undergoing DMSO treatment for a sprained wrist may have been due to the treatment, although no autopsy was done, nor was a causal relationship established.
This neurotoxicity could be detected at doses as low as 0.3 mL/kg, a level exceeded in children exposed to DMSO during bone marrow transplant.
[51] However, chemically pure DMSO is odorless because of the lack of C-S-C (sulfide) and C-S-H (mercaptan) linkages.
[citation needed] DMSO can decompose at the boiling temperature of 189 °C at normal pressure, possibly leading to an explosion.