Dipole
(To be precise: for the definition of the dipole moment, one should always consider the "dipole limit", where, for example, the distance of the generating charges should converge to 0 while simultaneously, the charge strength should diverge to infinity in such a way that the product remains a positive constant.)
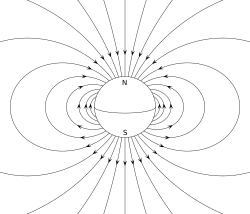
The two ends of a bar magnet are referred to as poles (not to be confused with monopoles, see Classification below) and may be labeled "north" and "south".
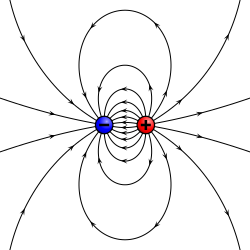
A physical dipole consists of two equal and opposite point charges: in the literal sense, two poles.
Many molecules have such dipole moments due to non-uniform distributions of positive and negative charges on the various atoms.
Such is the case with polar compounds like hydrogen fluoride (HF), where electron density is shared unequally between atoms.
The physical chemist Peter J. W. Debye was the first scientist to study molecular dipoles extensively, and, as a consequence, dipole moments are measured in the non-SI unit named debye in his honor.
For molecules there are three types of dipoles: More generally, an induced dipole of any polarizable charge distribution ρ (remember that a molecule has a charge distribution) is caused by an electric field external to ρ.
Some typical gas phase values given with the unit debye are:[7] Potassium bromide (KBr) has one of the highest dipole moments because it is an ionic compound that exists as a molecule in the gas phase.
As a vector sum it depends on the relative orientation of the bonds, so that from the dipole moment information can be deduced about the molecular geometry.
This agrees with the Lewis structures for the resonance forms of ozone which show a positive charge on the central oxygen atom.
An example in organic chemistry of the role of geometry in determining dipole moment is the cis and trans isomers of 1,2-dichloroethene.
However, due to the equilateral triangular distribution of the fluoride ions centered on and in the same plane as the boron cation, the symmetry of the molecule results in its dipole moment being zero.
Consider a collection of N particles with charges qi and position vectors ri.
The dipole observable (physical quantity) has the quantum mechanical dipole operator:[citation needed] Notice that this definition is valid only for neutral atoms or molecules, i.e. total charge equal to zero.
All 3 components of the dipole operator are antisymmetric under inversion with respect to the nucleus, where
Since the only quantity that is equal to minus itself is the zero, the expectation value vanishes, In the case of open-shell atoms with degenerate energy levels, one could define a dipole moment by the aid of the first-order Stark effect.
This gives a non-vanishing dipole (by definition proportional to a non-vanishing first-order Stark shift) only if some of the wavefunctions belonging to the degenerate energies have opposite parity; i.e., have different behavior under inversion.
This is a rare occurrence, but happens for the excited H-atom, where 2s and 2p states are "accidentally" degenerate (see article Laplace–Runge–Lenz vector for the origin of this degeneracy) and have opposite parity (2s is even and 2p is odd).
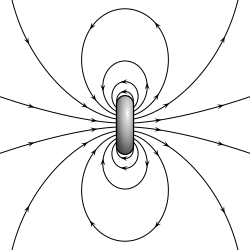
The far-field strength, B, of a dipole magnetic field is given by where Conversion to cylindrical coordinates is achieved using r2 = z2 + ρ2 and where ρ is the perpendicular distance from the z-axis.
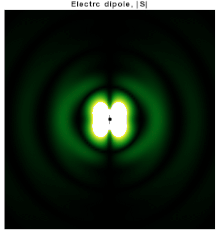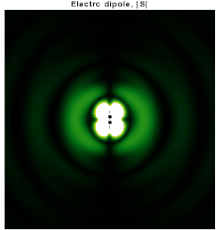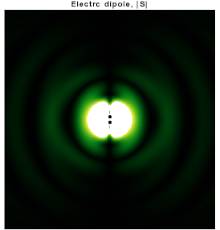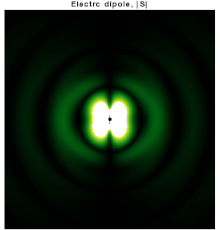
In particular, consider a harmonically oscillating electric dipole, with angular frequency ω and a dipole moment p0 along the ẑ direction of the form In vacuum, the exact field produced by this oscillating dipole can be derived using the retarded potential formulation as: For rω/c ≫ 1, the far-field takes the simpler form of a radiating "spherical" wave, but with angular dependence embedded in the cross-product:[10] The time-averaged Poynting vector is not distributed isotropically, but concentrated around the directions lying perpendicular to the dipole moment, as a result of the non-spherical electric and magnetic waves.
In fact, the spherical harmonic function (sin θ) responsible for such toroidal angular distribution is precisely the l = 1 "p" wave.