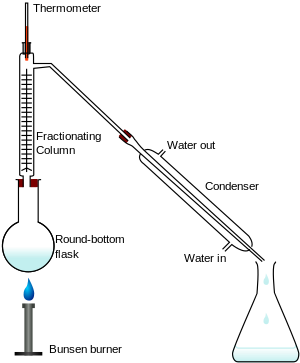
Fractionating column
A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close volatility.
The separation may be enhanced by the addition of more trays (to a practical limitation of heat, flow, etc.).
[1][2] Fractionating columns are widely used in chemical process industries where large quantities of liquids have to be distilled.
In such refineries, the crude oil feedstock is a complex, multicomponent mixture that must be separated.
Industrial fractionating columns use external reflux to achieve better separation of products.
[3][5] Reflux refers to the portion of the condensed overhead liquid product that returns to the upper part of the fractionating column as shown in Figure 3.
Inside the column, the downflowing reflux liquid provides cooling and condensation of upflowing vapors thereby increasing the efficacy of the distillation tower.
Bubble-cap "trays" or "plates" are one of the types of physical devices, which are used to provide good contact between the upflowing vapor and the downflowing liquid inside an industrial fractionating column.
Hence, a fractionating column almost always needs more actual, physical plates than the required number of theoretical vapor–liquid equilibrium stages.