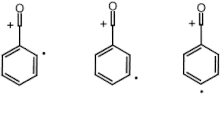
Distonic ion
Distonic ions are chemical species that contain ionic charges and radical sites in different locations (on separate atoms), unlike regular radicals where the formal charge and unpaired electron are in the same location.
[1] These molecular species are created by ionization of either zwitterions or diradicals; ultimately, a neutral molecule loses an electron.
In 1984 scientists Bouma, Radom and Yates originated the term through extensive experimental research but they were not the first to deal with distonic ions.
[8] Distonic ions have been extensively examined due to their unique behavior and how commonly they can occur.
[clarification needed][3] It may be difficult to decipher the functions[clarification needed] of the charge and radical site because distonic ions are limited to elementary reactions such as unimolecular reactions involving highly excited and short-lived species.