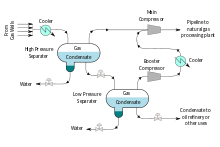
Natural-gas condensate
In general, gas condensate has a specific gravity ranging from 0.5 to 0.8, and is composed of hydrocarbons such as propane, butane, pentane, and hexane.
Then, the ethane (C2), propane (C3), butanes (C4), and pentanes (C5)—plus higher molecular weight hydrocarbons referred to as C5+—will also be removed and recovered as byproducts.
Some of the raw natural gas may be re-injected into the producing formation to help maintain the reservoir pressure, or for storage pending later installation of a pipeline.
[11][12] Drip gas is defined in the United States Code of Federal Regulations as consisting of butane, pentane, and hexane hydrocarbons.
By 1930, improved engines and higher compression ratios required higher-octane, refined gasolines to produce power without knocking or detonation.
Beginning in the Great Depression, drip gas was used as a replacement for commercial gasoline by people in oil-producing areas.
"In the days of simple engines in automobiles and farm tractors it was not uncommon for anyone having access to a condensate well to fill his tank with 'drip,'" according to the Oklahoma Historical Society.
Given the poor vaporization at low temperatures, all-fuel tractors were started on gasoline, then switched to the heavy fuel.
Shutters or curtains were typically used to restrict airflow to the radiator, keeping the engine sufficiently hot for efficient operation.
Natural convection allowed the water to flow up and out of the engine block and into the top of the radiator, where it cooled and dropped and fell to continue the cycle.
The white gas sold today is a similar product but is produced at refineries with the carcinogen benzene removed.
It is also harmful to modern engines due to its low octane rating, much higher combustion temperature than that of gasoline, and lack of additives.