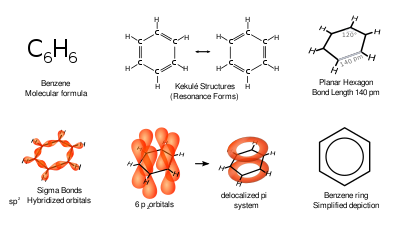Benzene
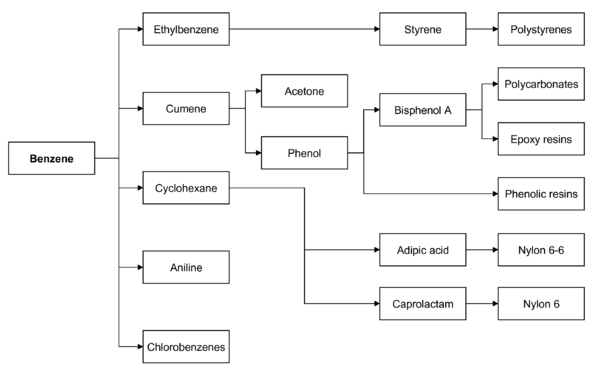
It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually.
Its particular effects on human health, such as the long-term results of accidental exposure, have been reported on by news organizations such as The New York Times.
[18] Michael Faraday first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name bicarburet of hydrogen.
In 1845, Charles Blachford Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar.
In 1865, the German chemist Friedrich August Kekulé published a paper in French (for he was then teaching in Francophone Belgium) suggesting that the structure contained a ring of six carbon atoms with alternating single and double bonds.
He said that he had discovered the ring shape of the benzene molecule after having a reverie or day-dream of a snake biting its own tail (a symbol in ancient cultures known as the ouroboros).
Curiously, a similar, humorous depiction of benzene had appeared in 1886 in a pamphlet entitled Berichte der Durstigen Chemischen Gesellschaft (Journal of the Thirsty Chemical Society), a parody of the Berichte der Deutschen Chemischen Gesellschaft, only the parody had monkeys seizing each other in a circle, rather than snakes as in Kekulé's anecdote.
Through calculating more than thirty parameters, Lonsdale demonstrated that the benzene ring could not be anything but a flat hexagon, and provided accurate distances for all carbon-carbon bonds in the molecule.
[48][49] It was the German chemist Carl Gräbe who, in 1869, first used the prefixes ortho-, meta-, para- to denote specific relative locations of the substituents on a di-substituted aromatic ring (viz, naphthalene).
For commercial use, until World War II, much of benzene was obtained as a by-product of coke production (or "coke-oven light oil") for the steel industry.
To reflect the delocalized nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms.
Derivatives of benzene occur sufficiently often as a component of organic molecules, so much so that the Unicode Consortium has allocated a symbol in the Miscellaneous Technical block with the code U+232C (⌬) to represent it with three double bonds,[63] and U+23E3 (⏣) for a delocalized version.
Recovery of the aromatics, commonly referred to as BTX (benzene, toluene and xylene isomers), involves such extraction and distillation steps.
In similar fashion to this catalytic reforming, UOP and BP commercialized a method from LPG (mainly propane and butane) to aromatics.
In this hydrogen-intensive process, toluene is mixed with hydrogen, then passed over a chromium, molybdenum, or platinum oxide catalyst at 500–650 °C and 20–60 atm pressure.
Under these conditions, toluene undergoes dealkylation to benzene and methane: This irreversible reaction is accompanied by an equilibrium side reaction that produces biphenyl (diphenyl) at higher temperature: If the raw material stream contains much non-aromatic components (paraffins or naphthenes), those are likely decomposed to lower hydrocarbons such as methane, which increases the consumption of hydrogen.
Cyclohexane consumes around 10% of the world's benzene production; it is primarily used in the manufacture of nylon fibers, which are processed into textiles and engineering plastics.
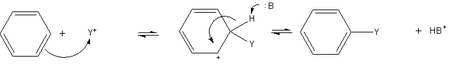
In nitration, benzene reacts with nitronium ions (NO2+), which is a strong electrophile produced by combining sulfuric and nitric acids.
Substantial quantities of epidemiologic, clinical, and laboratory data link benzene to aplastic anemia, acute leukemia, bone marrow abnormalities and cardiovascular disease.
Long-term exposure to excessive levels of benzene in the air causes leukemia, a potentially fatal cancer of the blood-forming organs.
Benzene targets the liver, kidney, lung, heart and brain and can cause DNA strand breaks and chromosomal damage, hence is teratogenic and mutagenic.
[83][84] According to the Agency for Toxic Substances and Disease Registry (ATSDR) (2007), benzene is both a synthetically made and naturally occurring chemical from processes that include: volcanic eruptions, wild fires, synthesis of chemicals such as phenol, production of synthetic fibers, and fabrication of rubbers, lubricants, pesticides, medications, and dyes.
Measurement of air and water levels of benzene is accomplished through collection via activated charcoal tubes, which are then analyzed with a gas chromatograph.
Benzene itself can be measured in breath, blood or urine, but such testing is usually limited to the first 24 hours post-exposure due to the relatively rapid removal of the chemical by exhalation or biotransformation.
In bacteria, dioxygenase enzyme can add an oxygen to the ring, and the unstable product is immediately reduced (by NADH) to a cyclic diol with two double bonds, breaking the aromaticity.
These intermediates and metabolites induce genotoxicity by multiple mechanisms including inhibition of topoisomerase II (which maintains chromosome structure), disruption of microtubules (which maintains cellular structure and organization), generation of oxygen free radicals (unstable species) that may lead to point mutations, increasing oxidative stress, inducing DNA strand breaks, and altering DNA methylation (which can affect gene expression).
These aberrations can be monitored using fluorescent in situ hybridization (FISH) with DNA probes to assess the effects of benzene along with the hematological tests as markers of hematotoxicity.
Individuals carrying variant of NAD(P)H:quinone oxidoreductase 1 (NQO1), microsomal epoxide hydrolase (EPHX) and deletion of the glutathione S-transferase T1 (GSTT1) showed a greater frequency of DNA single-stranded breaks.
In March 2006, the official Food Standards Agency in United Kingdom conducted a survey of 150 brands of soft drinks.
[108] In 2005, the water supply to the city of Harbin in China with a population of almost nine million people, was cut off because of a major benzene exposure.