Dye-sensitized solar cell
[5] The DSSC has a number of attractive features; it is simple to make using conventional roll-printing techniques, is semi-flexible and semi-transparent which offers a variety of uses not applicable to glass-based systems, and most of the materials used are low-cost.
In practice it has proven difficult to eliminate a number of expensive materials, notably platinum and ruthenium, and the liquid electrolyte presents a serious challenge to making a cell suitable for use in all weather.
Although its conversion efficiency is less than the best thin-film cells, in theory its price/performance ratio should be good enough to allow them to compete with fossil fuel electrical generation by achieving grid parity.
Commercial applications, which were held up due to chemical stability problems,[6] had been forecast in the European Union Photovoltaic Roadmap to significantly contribute to renewable electricity generation by 2020.
[10] In an effort to understand and simulate the primary processes in photosynthesis the phenomenon was studied at the University of California at Berkeley with chlorophyll extracted from spinach (bio-mimetic or bionic approach).
As stated before, the counter electrode is responsible for collecting electrons from the external circuit and introducing them back into the electrolyte to catalyze the reduction reaction of the redox shuttle, generally I3− to I−.
One such category being widely studied includes chalcogen compounds of cobalt, nickel, and iron (CCNI), particularly the effects of morphology, stoichiometry, and synergy on the resulting performance.
Because a material's electrocatalytic potential is highly dependent on the amount of surface area available to facilitate the diffusion and reduction of the redox species, numerous research efforts have been focused towards understanding and optimizing the morphology of nanostructures for DSSC counter electrodes.
[19] Comparison of these three morphologies revealed that the hybrid composite nanoparticles, due to having the largest electroactive surface area, had the highest power conversion efficiency of 9.27%, even higher than its platinum counterpart.
With a similar study but a different system, Du et al. in 2017 determined that the ternary oxide of NiCo2O4 had the greatest power conversion efficiency and electrocatalytic ability as nanoflowers when compared to nanorods or nanosheets.
[20] Du et al. realized that exploring various growth mechanisms that help to exploit the larger active surface areas of nanoflowers may provide an opening for extending DSSC applications to other fields.
Of course, the composition of the material that is used as the counter electrode is extremely important to creating a working photovoltaic, as the valence and conduction energy bands must overlap with those of the redox electrolyte species to allow for efficient electron exchange.
[21] Nickel and cobalt bimetallic alloys were known to have outstanding electron conduction and stability, so optimizing its stoichiometry would ideally produce a more efficient and stable cell performance than its singly metallic counterparts.
Such is the result that Jin et al. found, as Ni0.12Co0.80Se achieved superior power conversion efficiency (8.61%), lower charge transfer impedance, and higher electrocatalytic ability than both its platinum and binary selenide counterparts.
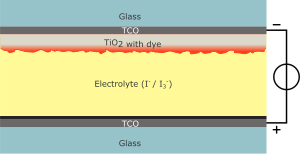
The injected electron diffuses through the sintered particle network to be collected at the front side transparent conducting oxide (TCO) electrode, while the dye is regenerated via reduction by a redox shuttle, I3−/I−, dissolved in a solution.
In theory, the maximum voltage generated by such a cell is simply the difference between the (quasi-)Fermi level of the TiO2 and the redox potential of the electrolyte, about 0.7 V under solar illumination conditions (Voc).
Typically used dye molecules generally have poorer absorption in the red part of the spectrum compared to silicon, which means that fewer of the photons in sunlight are usable for current generation.
DSSCs are therefore able to work under cloudy skies and non-direct sunlight, whereas traditional designs would suffer a "cutout" at some lower limit of illumination, when charge carrier mobility is low and recombination becomes a major issue.
The fragility of traditional silicon cells requires them to be protected from the elements, typically by encasing them in a glass box similar to a greenhouse, with a metal backing for strength.
Researchers have found that using dyes comprising a perylenemonoimide (PMI) as the acceptor and an oligothiophene coupled to triphenylamine as the donor greatly improve the performance of p-DSC by reducing charge recombination rate following dye-sensitized hole injection.
Newer versions were quickly introduced (circa 1999) that had much wider frequency response, notably "triscarboxy-ruthenium terpyridine" [Ru(4,4',4"-(COOH)3-terpy)(NCS)3], which is efficient right into the low-frequency range of red and IR light.
The newer dyes included 1-ethyl-3 methylimidazolium tetrocyanoborate [EMIB(CN)4] which is extremely light- and temperature-stable, copper-diselenium [Cu(In,GA)Se2] which offers higher conversion efficiencies, and others with varying special-purpose properties.
A group of researchers at the École Polytechnique Fédérale de Lausanne (EPFL) has reportedly increased the thermostability of DSC by using amphiphilic ruthenium sensitizer in conjunction with quasi-solid-state gel electrolyte.
Dyesol Director Gordon Thompson said, "The materials developed during this joint collaboration have the potential to significantly advance the commercialisation of DSC in a range of applications where performance and stability are essential requirements.
Michael Grätzel announced the fabrication of Solid State DSSCs with 15.0% efficiency, reached by the means of a hybrid perovskite CH3NH3PbI3 dye, subsequently deposited from the separated solutions of CH3NH3I and PbI2.
[43][42] The field of building-integrated photovoltaics (BIPV) has gained attention from the scientific community due to its potential to reduce pollution and materials and electricity costs, as well as to improve the aesthetics of a building.
[69] In recent years, scientists have looked at ways to incorporate DSSC’s in BIPV applications, since the dominant Si-based PV systems in the market have a limited presence in this field due to their energy-intensive manufacturing methods, poor conversion efficiency under low light intensities, and high maintenance requirements.
The motivation for this change was that, despite that glass substrates have resulted in the highest recorded efficiencies for DSSC’s, for BIPV applications like roof tiles or building facades, lighter and more flexible materials are essential.
[73] The researchers from Switzerland’s École polytechnique fédérale de Lausanne (EPFL) found that the efficiency to cosensitized solar cells can be raised by the pre-adsorption of a monolayer of hydroxamic acid derivative on a surface of nanocrystalline mesoporous titanium dioxide, which functions as the electron transport mechanism of the electrode.
[74] The DSSC developed by the team showed a record-breaking power conversion efficiency of 15.2% under standard global simulated sunlight and long-term operational stability over 500 hours.