Ectopic pregnancy
[5] Risk factors for ectopic pregnancy include pelvic inflammatory disease, often due to chlamydia infection; tobacco smoking; endometriosis; prior tubal surgery; a history of infertility; and the use of assisted reproductive technology.
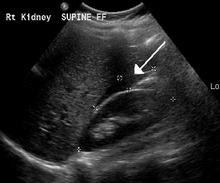
[1] Detection of ectopic pregnancy is typically by blood tests for human chorionic gonadotropin (hCG) and ultrasound.
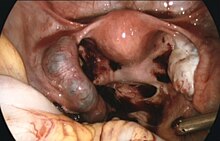
[2] Surgery such as a salpingectomy is still typically recommended if the tube has ruptured, there is a fetal heartbeat, or the woman's vital signs are unstable.
[2] The rate of ectopic pregnancy is about 11 to 20 per 1,000 live births in developed countries, though it may be as high as 4% among those using assisted reproductive technology.
[5] In many cases the symptoms have low specificity, and can be similar to those of other genitourinary and gastrointestinal disorders, such as appendicitis, salpingitis, rupture of a corpus luteum cyst, miscarriage, ovarian torsion or urinary tract infection.
[5] Rupture of an ectopic pregnancy can lead to symptoms such as abdominal distension, tenderness, peritonism and hypovolemic shock.
[19] The exact mechanism through which chlamydia increases the risk of ectopic pregnancy is uncertain, though some research suggests that the infection can affect the structure of fallopian tubes.
The fertilized egg, if it does not reach the uterus in time, will hatch from the non-adhesive zona pellucida and implant itself inside the fallopian tube, thus causing the ectopic pregnancy.
[citation needed] Women with pelvic inflammatory disease (PID) have a high occurrence of ectopic pregnancy.
The higher endometriosis risks were attributed to increased pelvic inflammation and structural and functional changes in the uterus' lining.
[30] It has also been suggested that pathologic generation of nitric oxide through increased iNOS production may decrease tubal ciliary beats and smooth muscle contractions and thus affect embryo transport, which may consequently result in ectopic pregnancy.
[5] An ultrasound showing a gestational sac with fetal heart in the fallopian tube has a very high specificity of ectopic pregnancy.
[5] A small amount of anechogenic-free fluid in the recto-uterine pouch is commonly found in both intrauterine and ectopic pregnancies.
[5] Where no intrauterine pregnancy (IUP) is seen on ultrasound, measuring β-human chorionic gonadotropin (β-hCG) levels may aid in the diagnosis.
[citation needed] Culdocentesis, in which fluid is retrieved from the space separating the vagina and rectum, is a less commonly performed test that may be used to look for internal bleeding.
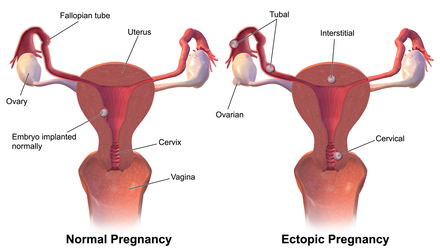
Specific indications for this procedure include either of the following:[5] The vast majority of ectopic pregnancies implant in the fallopian tube.
[43][44] CSP are characterized by abnormal implantation into the scar from a previous cesarean section,[45] and allowed to continue can cause serious complications such as uterine rupture and hemorrhage.
[45][48][49] Given the rarity of the diagnosis, treatment options tend to be described in case reports and series, ranging from medical with methotrexate or KCl[50] to surgical with dilation and curettage,[51] uterine wedge resection,[citation needed] or hysterectomy.
This type of ectopic pregnancy is often results in rupture of the rudimentary horn between 10 and 15 weeks of gestation, leading to a high risk of morbidity and mortality.
For this reason hCG levels may have to be monitored after removal of an ectopic pregnancy to assure their decline, also methotrexate can be given at the time of surgery prophylactically.
[5] Persisting PUL is where the hCG level does not spontaneously decline and no intrauterine or ectopic pregnancy is identified on follow-up transvaginal ultrasonography.
[5] A treated persistent PUL is defined as one managed medically (generally with methotrexate) without confirmation of the location of the pregnancy such as by ultrasound, laparoscopy or uterine evacuation.
[5] A resolved persistent PUL is defined as serum hCG reaching a non-pregnant value (generally less than 5 IU/L) after expectant management, or after uterine evacuation without evidence of chorionic villi on histopathological examination.
Contraindications include ectopic embryonic mass > 3.5 cm and evidence of ruptured fallopian tube, as well as renal or hepatic dysfunction.
[5] Therefore, it is recommended that methotrexate should only be administered when hCG has been serially monitored with a rise less than 35% over 48 hours, which practically excludes a viable intrauterine pregnancy.
However, whether to pursue surgical intervention is an often difficult decision in a stable patient with minimal evidence of blood clot on ultrasound.
[64] When ectopic pregnancies are treated, the prognosis for the mother is very good in Western countries; maternal death is rare, but all babies die or are aborted.
[5] Salpingectomy as a treatment for ectopic pregnancy is one of the common cases when the principle of double effect can be used to justify accelerating the death of the embryo by doctors and patients opposed to outright abortions.
In July 1999, Lori Dalton gave birth by caesarean section in Ogden, Utah, United States, to a healthy baby girl, Saige, who had developed outside of the uterus.
[81] On May 29, 2008, an Australian woman, Meera Thangarajah (age 34), who had an ectopic pregnancy in the ovary, gave birth to a healthy full term 6 pound 3 ounce (2.8 kg) baby girl, Durga, via Caesarean section.