Einsteinium
Its most common isotope, einsteinium-253 (253Es; half-life 20.47 days), is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year.
Due to the small amounts of einsteinium produced and the short half-life of its most common isotope, there are no practical applications for it except basic scientific research.
The high radioactivity of 253Es produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watts per gram.
The longest-lived isotope of einsteinium, 252Es (half-life 471.7 days) would be more suitable for investigation of physical properties, but it has proven far more difficult to produce and is available only in minute quantities, not in bulk.
[5] Einsteinium was first identified in December 1952 by Albert Ghiorso and co-workers at University of California, Berkeley in collaboration with the Argonne and Los Alamos National Laboratories, in the fallout from the Ivy Mike nuclear test.
[6] Initial examination of the debris from the explosion had shown the production of a new isotope of plutonium, 24494Pu, which could only have formed by the absorption of six neutrons by a uranium-238 nucleus followed by two beta decays.
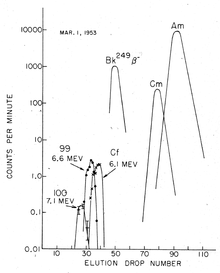
[6] Ghiorso and co-workers analyzed filter papers which had been flown through the explosion cloud on airplanes (the same sampling technique that had been used to discover 244Pu).
[7] Larger amounts of radioactive material were later isolated from coral debris of the atoll, and these were delivered to the U.S.[6] The separation of suspected new elements was carried out in the presence of a citric acid/ammonium buffer solution in a weakly acidic medium (pH ≈ 3.5), using ion exchange at elevated temperatures; fewer than 200 atoms of einsteinium were recovered in the end.
[9] The discovery of the new elements and the associated new data on multiple neutron capture were initially kept secret on the orders of the U.S. military until 1955 due to Cold War tensions and competition with Soviet Union in nuclear technologies.
In late 1953 – early 1954, the Swedish group succeeded in synthesizing light isotopes of element 100, in particular 250Fm, by bombarding uranium with oxygen nuclei.
[28] Unlike the lighter actinides californium, berkelium, curium and americium, which crystallize in a double hexagonal structure at ambient conditions; einsteinium is believed to have a face-centered cubic (fcc) symmetry with the space group Fm3m and the lattice constant a = 575 pm.
Other experimental techniques to circumvent the contamination problem include selective optical excitation of einsteinium ions by a tunable laser, such as in studying its luminescence properties.
[42] The existence of divalent einsteinium is firmly established, especially in the solid phase; such +2 state is not observed in many other actinides, including protactinium, uranium, neptunium, plutonium, curium and berkelium.
Synthesis of einsteinium from naturally-occurring uranium and thorium in the Earth's crust requires multiple neutron capture, an extremely unlikely event.
[8] The transuranic elements americium to fermium, including einsteinium, were once created in the natural nuclear fission reactor at Oklo, but any quantities produced then would have long since decayed away.
[48] However, the lead author of the studies finding einsteinium and other short-lived actinides in Przybylski's Star, Vera F. Gopka, admitted that "the position of lines of the radioactive elements under search were simply visualized in synthetic spectrum as vertical markers because there are not any atomic data for these lines except for their wavelengths (Sansonetti et al. 2004), enabling one to calculate their profiles with more or less real intensities.
Einsteinium is produced in minute quantities by bombarding lighter actinides with neutrons in dedicated high-flux nuclear reactors.
These facilities have similar power and flux levels, and are expected to have comparable production capacities for transcurium elements,[54] though the quantities produced at NIIAR are not widely reported.
In a "typical processing campaign" at ORNL, tens of grams of curium are irradiated to produce decigram quantities of californium, milligrams of berkelium (249Bk) and einsteinium and picograms of fermium.
[65] 253Es was produced by irradiating a 0.1–0.2 milligram 252Cf target with a thermal neutron flux of (2–5)×1014 neutrons/(cm2·s) for 500–900 hours:[66] In 2020, scientists at ORNL created about 200 nanograms of 254Es; allowing some chemical properties of the element to be studied for the first time.
Aircraft filters adsorbed only ~4×10−14 of the total amount, and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude.
This method was tried in two tests and instantly provided hundreds of kilograms of material, but with actinide concentration 3 times lower than in samples obtained after drilling.
[74] The yields are much higher for reactor irradiation, but there, the product is a mixture of various actinide isotopes, as well as lanthanides produced in the nuclear fission decays.
In this case, isolation of einsteinium is a tedious procedure which involves several repeating steps of cation exchange, at elevated temperature and pressure, and chromatography.
Separation from berkelium is important, because the most common einsteinium isotope produced in nuclear reactors, 253Es, decays with a half-life of only 20 days to 249Bk, which is fast on the timescale of most experiments.
[75] Trivalent actinides can be separated from lanthanide fission products by a cation-exchange resin column using a 90% water/10% ethanol solution saturated with hydrochloric acid (HCl) as eluant.
An alternative preparation procedure is to exposure Es(III) oxide to chlorine trifluoride (ClF3) or F2 gas at a pressure of 1–2 atmospheres and temperature 300–400°C.
[98] The rare isotope 254Es is favored for production of superheavy elements due to its large mass, relatively long half-life of 270 days, and availability in significant amounts of several micrograms.
[99] Hence 254Es was used as a target in the attempted synthesis of ununennium (element 119) in 1985 by bombarding it with calcium-48 ions at the superHILAC linear particle accelerator at Berkeley, California.
The large mass of this isotope reduced the spectral overlap between signals from the marker and the studied lighter elements of the lunar surface.