Electrolyte
Some gases, such as hydrogen chloride (HCL), under conditions of high temperature or low pressure can also function as electrolytes.
[clarification needed] Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) or synthetic polymers (e.g., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups.
In medicine, electrolyte replacement is needed when a person has prolonged vomiting or diarrhea, and as a response to sweating due to strenuous athletic activity.
[2] In clinical medicine, mentions of electrolytes usually refer metonymically to the ions, and (especially) to their concentrations (in blood, serum, urine, or other fluids).
[citation needed] In his 1884 dissertation, Svante Arrhenius put forth his explanation of solid crystalline salts disassociating into paired charged particles when dissolved, for which he won the 1903 Nobel Prize in Chemistry.
[7][8][9][10] Arrhenius's explanation was that in forming a solution, the salt dissociates into charged particles, to which Michael Faraday (1791-1867) had given the name "ions" many years earlier.
In particular, ionic liquids, which are molten salts with melting points below 100 °C,[15] are a type of highly conductive non-aqueous electrolytes and thus have found more and more applications in fuel cells and batteries.
[19][failed verification] The electric charge symbols of plus (+) and minus (−) indicate that the substance is ionic in nature and has an imbalanced distribution of electrons, the result of chemical dissociation.
Such gradients affect and regulate the hydration of the body as well as blood pH, and are critical for nerve and muscle function.
[citation needed][23] Electrolyte balance is maintained by oral, or in emergencies, intravenous (IV) intake of electrolyte-containing substances, and is regulated by hormones, in general with the kidneys flushing out excess levels.
Serious electrolyte disturbances, such as dehydration and overhydration, may lead to cardiac and neurological complications and, unless they are rapidly resolved, will result in a medical emergency.
The interpretation of these values is somewhat meaningless without analysis of the clinical history and is often impossible without parallel measurements of renal function.
[citation needed] According to a study paid for by the Gatorade Sports Science Institute, electrolyte drinks containing sodium and potassium salts replenish the body's water and electrolyte concentrations after dehydration caused by exercise, excessive alcohol consumption, diaphoresis (heavy sweating), diarrhea, vomiting, intoxication or starvation; the study says that athletes exercising in extreme conditions (for three or more hours continuously, e.g. a marathon or triathlon) who do not consume electrolytes risk dehydration (or hyponatremia).
[24][needs independent confirmation] A home-made electrolyte drink can be made by using water, sugar and salt in precise proportions.
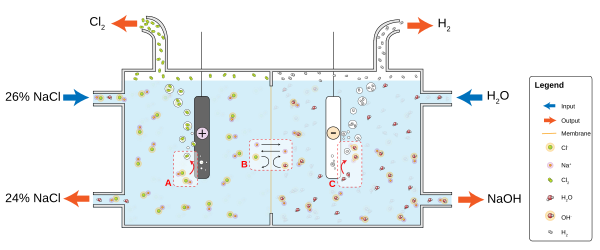
Electrolytic conductors are used in electronic devices where the chemical reaction at a metal-electrolyte interface yields useful effects.
In order to increase the mechanical strength and conductivity of such electrolytes, very often composites are made, and inert ceramic phase is introduced.
[30][31][32] Organic ionic plastic crystals – are a type organic salts exhibiting mesophases (i.e. a state of matter intermediate between liquid and solid), in which mobile ions are orientationally or rotationally disordered while their centers are located at the ordered sites in the crystal structure.
[28] They have various forms of disorder due to one or more solid–solid phase transitions below the melting point and have therefore plastic properties and good mechanical flexibility as well as an improved electrode-electrolyte interfacial contact.