Electron ionization
Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods.
[5] Electron ionization was first described in 1918 by Canadian-American Physicist Arthur J. Dempster in the article of "A new method of positive ray analysis."
The use of a focused monoenergetic beam of electrons for ionization of gas phase atoms and molecules was developed by Bleakney in 1929.
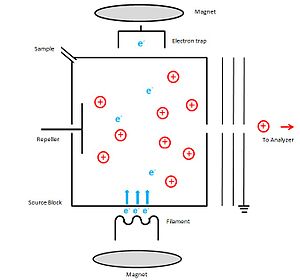
In an EI ion source, electrons are produced through thermionic emission by heating a wire filament that has electric current running through it.
The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular orientation to the electron beam.
The equation can be shown as follows: The ion extraction efficiency (β) can be optimized by increasing the voltage of both repeller and acceleration.
Since the ionization cross section depends on the chemical nature of the sample and the energy of ionizing electrons a standard value of 70 eV is used.
A potential of 70 V is applied between the cathode and source block to accelerate them to 70 eV kinetic energy to produce positive ions.
The potential of the anode (electron trap) is slightly positive and it is placed on the outside of the ionization chamber, directly opposite to the cathode.
To increase the ionization process, a weak magnetic field is applied parallel to the direction of the electrons' travel.
GC can be incorporated for the separation of mixtures of thermally stable and volatile gases which are in perfect match with the electron ionization conditions.
From this analysis scientists found that the material used to waterproof the amphorae was a particular type of resin not native to the archaeological site but imported from another region.
This method is fast, simple and cost effective since high numbers of pesticides can be determined by GC with a single injection, considerably reducing the total time for the analysis.
This investigation used a new rapid and sensitive electron ionization-gas chromatography–mass spectrometry method in selective ion monitoring mode (SIM) with a single injection of the sample.
The detection of specific residues in blood is a difficult task due to their very low concentration since as soon as they enter the body most of the chemicals may get excreted.
This method is very efficient since both free and protein-bound d-phenylalanine can be measured using the same mass spectrometer and only a small amount of protein is needed (about 1 mg).
One example is the analysis of five local anesthetics in blood using headspace solid-phase microextraction (HS-SPME) and gas chromatography–mass spectrometry–electron impact ionization selected ion monitoring (GC–MS–EI-SIM).
The analyses of these drugs are difficult due to the low concentrations in the body fluids and often a long time delay between the event and clinical examination.
[20] Two recent approaches for coupling capillary scale liquid chromatography-electron ionization mass spectrometry (LC-EI-MS) can be incorporated for the analysis of various samples.
According to this category most of the time applications can be found in time of flight (TOF) or orthogonal TOF mass spectrometry (OA-TOF MS), Fourier transform ion cyclotron resonance (FT-ICR MS) and quadrupole or ion trap mass spectrometry.
The electron ionization time of flight mass spectroscopy (EI-TOF MS) is well suited for analytical and basic chemical physics studies.
Autodetachment lifetimes, metastable dissociation, Rydberg electron transfer reactions and field detachment, SF6 scavenger method for detecting temporary negative ion states, and many others have all been discovered using this technique.
[23] FT- ICR EI - MS can be used for analysis of three vacuum gas oil (VGO) distillation fractions in 295-319 °C, 319-456 °C and 456-543 °C.
In this method, EI at 10 eV allows soft ionization of aromatic compounds in the vacuum gas oil range.
Ultra-high resolving power, small sample size, high reproducibility and mass accuracy (<0.4ppm) are the special features in this method.
In addition, many sulfur-, nitrogen-, and oxygen-containing compounds were directly observed when the concentration of this heteroatomic species increased with the boiling point.
Using data analysis it gave the information about compound types (rings plus double bonds), their carbon number distributions for hydrocarbon and heteroatomic compounds in the distillation fractions, increasing average molecular weight (or carbon number distribution) and aromaticity with increasing boiling temperature of the petroleum fractions.
Form this research, they have found out that the ion trap GC- MS is a reliable and convenient analytical approach with variety of ionization methods including EI, for the determination of target compounds in environmental samples.