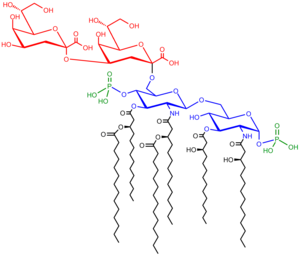
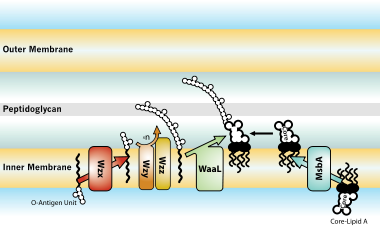
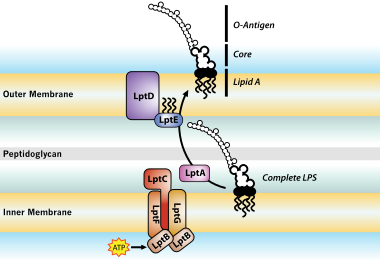
Lipopolysaccharide
[4] In severe cases, LPS can trigger a brisk host response and multiple types of acute organ failure [5] which can lead to septic shock.
[6] In lower levels and over a longer time period, there is evidence LPS may play an important and harmful role in autoimmunity, obesity, depression, and cellular senescence.
[11] Subsequent work showed that release of LPS from Gram negative microbes does not necessarily require the destruction of the bacterial cell wall, but rather, LPS is secreted as part of the normal physiological activity of membrane vesicle trafficking in the form of bacterial outer membrane vesicles (OMVs), which may also contain other virulence factors and proteins.
[13] It has also been implicated in non-pathogenic aspects of bacterial ecology, including surface adhesion, bacteriophage sensitivity, and interactions with predators such as amoebae.
In its place is a short oligosaccharide: this form is known as Lipooligosaccharide (LOS), and is a glycolipid found in the outer membrane of some types of Gram-negative bacteria, such as Neisseria spp.
[7][22] LOS plays a central role in maintaining the integrity and functionality of the outer membrane of the Gram negative cell envelope.
In the case of Neisseria meningitidis, the lipid A portion of the molecule has a symmetrical structure and the inner core is composed of 3-deoxy-D-manno-2-octulosonic acid (KDO) and heptose (Hep) moieties.
[7][22] A highly conserved host enzyme called acyloxyacyl hydrolase (AOAH) may detoxify LPS when it enters, or is produced in, animal tissues.
Dephosphorylation of LPS by intestinal alkaline phosphatase can reduce the severity of Salmonella tryphimurium and Clostridioides difficile infection restoring normal gut microbiota.
[34] The epithelial surfaces are colonized by a complex microbial flora (including gram-negative bacteria), which outnumber human cells by a factor of 10 to 1.
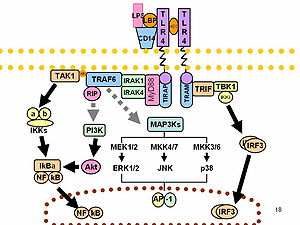
[36][37] As part of the cellular stress response, superoxide is one of the major reactive oxygen species induced by LPS in various cell types that express TLR (toll-like receptor).
[41][42] It may also be linked to multiple immune tactics against pathogens, and part of a multi-faceted anti-microbial strategy that has been informed by human behavioral changes over our species' evolution (e.g., meat eating, agricultural practices, and smoking).
[7] LPS can be sensed directly by hematopoietic stem cells (HSCs) through the bonding with TLR4, causing them to proliferate in reaction to a systemic infection.
[47][48] In general, LPS gene clusters are highly variable between different strains, subspecies, species of bacterial pathogens of plants and animals.
[7] Haemophilus somnus, a pathogen of cattle, has also been shown to display LOS phase variation, a characteristic which may help in the evasion of bovine host immune defenses.
[52] Taken together, these observations suggest that variations in bacterial surface molecules such as LOS can help the pathogen evade both the humoral (antibody and complement-mediated) and the cell-mediated (killing by neutrophils, for example) host immune defenses.
Recently, it was shown that in addition to TLR4 mediated pathways, certain members of the family of the transient receptor potential ion channels recognize LPS.
[57] Lipopolysaccharide is a significant factor that makes bacteria harmful, and it helps categorize them into different groups based on their structure and function.
Additionally, the nature of LPS, which has both water-attracting and water-repelling properties (amphiphilic), makes it challenging to develop sensitive and user-friendly tests.
This assay uses the biological response of the neutrophils in a patient’s blood to an immunological complex of endotoxin and exogenous antibody – the chemiluminescent reaction formed creates an emission of light.
High levels of LPS in the blood can lead to metabolic syndrome, increasing the risk of conditions like diabetes, heart disease, and liver problems.
[58] LPS also plays a crucial role in symptoms caused by infections from harmful bacteria, including severe conditions like Waterhouse-Friderichsen syndrome, meningococcemia, and meningitis.
[58] In general the health effects of LPS are due to its abilities as a potent activator and modulator of the immune system, especially its inducement of inflammation.
Complement activation and a rising anti-inflammatory response can lead to immune cell dysfunction, immunosuppression, widespread coagulopathy, serious tissue damage and can progress to multi-system organ failure and death.
[65] Lipid A may cause uncontrolled activation of mammalian immune systems with production of inflammatory mediators that may lead to endotoxic septic shock.
[66] Uncontrolled complement activation may trigger destructive endothelial damage leading to disseminated intravascular coagulation (DIC), or atypical hemolytic uremic syndrome (aHUS) with injury to various organs such as including kidneys and lungs.
[67] The skin can show the effects of vascular damage often coupled with depletion of coagulation factors in the form of petechiae, purpura and ecchymoses.
[8][70][71] Other studies have shown that purified endotoxin from Escherichia coli can induce obesity and insulin-resistance when injected into germ-free mouse models.
[72] A more recent study has uncovered a potentially contributing role for Enterobacter cloacae B29 toward obesity and insulin resistance in a human patient.
Commercially available ovalbumin that is contaminated with LPS can falsify research results, as it does not accurately reflect the effect of the protein antigen on animal physiology.