Histrionicotoxins
Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the family Dendrobatidae, notably Oophaga histrionica (formerly Dendrobates histrionicus), which are native to Colombia.
[2][3] They are notably less toxic than other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.
An account from his diary reads: "[...] called rana de veneno by the Spanish, about three inches long, yellow on the back, with very large black eyes... those who use poison catch the frogs in the woods and confine them in a hollow cane where they regularly feed them until they want the poison, when they take the unfortunate reptile and pass a pointed piece of wood down his throat and out of one of his legs.
Afterwards, below this white substance, appears a yellow oil, which is carefully scraped off, and retains its deadly influence for four to six months, according to the goodness (as they say) of the frog.
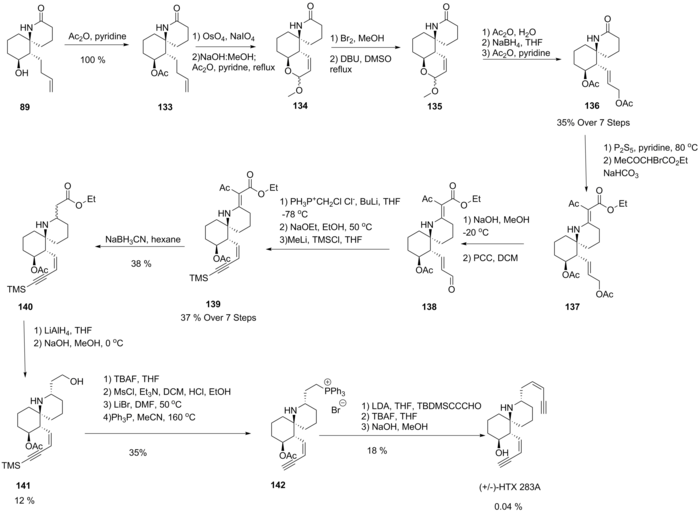
A Wittig reaction then generated a chloroalkene, which, upon base-promoted elimination of HCl, gave a terminal alkyne, which was subsequently protected to form 139.
A retro-Michael addition was then performed under basic conditions at low temperature, successfully epimerising this compound to give the desired epimer 141.
[12] The binding of histrionicotoxin is rapidly reversible, and so it can be readily removed from affected regions with repeated washing, or, in vivo, with natural bodily diffusion.