Energy density
In order of the typical magnitude of the energy stored, examples of reactions are: nuclear, chemical (including electrochemical), electrical, pressure, material deformation or in electromagnetic fields.
Liquid hydrocarbons (fuels such as gasoline, diesel and kerosene) are today the densest way known to economically store and transport chemical energy at a large scale (1 kg of diesel fuel burns with the oxygen contained in ≈ 15 kg of air).
Burning local biomass fuels supplies household energy needs (cooking fires, oil lamps, etc.)
Electrochemical reactions are used by devices such as laptop computers and mobile phones to release energy from batteries.
For example, the energy density of a magnetic field may be expressed as and behaves like a physical pressure.
When discussing the chemical energy contained, there are different types which can be quantified depending on the intended purpose.
One is the theoretical total amount of thermodynamic work that can be derived from a system, at a given temperature and pressure imposed by the surroundings, called exergy.
Another is the theoretical amount of electrical energy that can be derived from reactants that are at room temperature and atmospheric pressure.
There are two kinds of heat of combustion: A convenient table of HHV and LHV of some fuels can be found in the references.
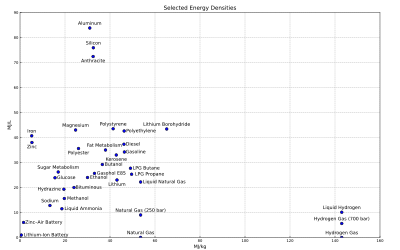
The adjacent figure shows the gravimetric and volumetric energy density of some fuels and storage technologies (modified from the Gasoline article).
This explains the apparently lower energy density of materials that contain their own oxidizer (such as gunpowder and TNT), where the mass of the oxidizer in effect adds weight, and absorbs some of the energy of combustion to dissociate and liberate oxygen to continue the reaction.
This also explains some apparent anomalies, such as the energy density of a sandwich appearing to be higher than that of a stick of dynamite.
Peukert's law describes how the amount of useful energy that can be obtained (for a lead-acid cell) depends on how quickly it is pulled out.
Fusion is the process by which the sun produces energy which will be available for billions of years (in the form of sunlight and heat).
A 1 inch tall uranium fuel pellet is equivalent to about 1 ton of coal, 120 gallons of crude oil, or 17,000 cubic feet of natural gas.
This represents a considerable density of energy that requires a continuous water flow at high velocity at all times in order to remove heat from the core, even after an emergency shutdown of the reactor.
Even in the case of relatively small black holes (smaller than astronomical objects) the power output would be tremendous.
In the case of absence of magnetic fields, by exploiting Fröhlich's relationships it is also possible to extend these equations to anisotropic and nonlinear dielectrics, as well as to calculate the correlated Helmholtz free energy and entropy densities.
When used to produce electricity in a fuel cell or to do work, it is the Gibbs free energy of reaction (ΔG) that sets the theoretical upper limit.
The mechanical energy storage capacity, or resilience, of a Hookean material when it is deformed to the point of failure can be computed by calculating tensile strength times the maximum elongation dividing by two.