Silicon
Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others.
These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating.
It is widely distributed throughout space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide (silica) or silicates.
Silicates are used in Portland cement for mortar and stucco, and mixed with silica sand and gravel to make concrete for walkways, foundations, and roads.
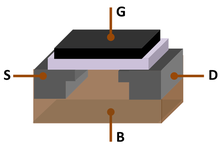
The small portion of very highly purified elemental silicon used in semiconductor electronics (<15%) is essential to the transistors and integrated circuit chips used in most modern technology such as smartphones and other computers.
Only traces are required by most animals, but some sea sponges and microorganisms, such as diatoms and radiolaria, secrete skeletal structures made of silica.
[20] Most other languages use transliterated forms of Davy's name, sometimes adapted to local phonology (e.g. German Silizium, Turkish silisyum, Catalan silici, Armenian Սիլիցիում or Silitzioum).
[32][33] Meanwhile, research on the chemistry of silicon continued; Friedrich Wöhler discovered the first volatile hydrides of silicon, synthesising trichlorosilane in 1857 and silane itself in 1858, but a detailed investigation of the silanes was only carried out in the early 20th century by Alfred Stock, despite early speculation on the matter dating as far back as the beginnings of synthetic organic chemistry in the 1830s.
Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of argon by forming sp3 hybrid orbitals, forming tetrahedral SiX4 derivatives where the central silicon atom shares an electron pair with each of the four atoms it is bonded to.
The hexacoordinate ionic radius of silicon may be considered to be 40 pm, although this must be taken as a purely notional figure given the lack of a simple Si4+ cation in reality.
However, doping silicon with a pnictogen such as phosphorus, arsenic, or antimony introduces one extra electron per dopant and these may then be excited into the conduction band either thermally or photolytically, creating an n-type semiconductor.
Similarly, doping silicon with a group 13 element such as boron, aluminium, or gallium results in the introduction of acceptor levels that trap electrons that may be excited from the filled valence band, creating a p-type semiconductor.
[51] Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic crystal lattice (space group 227).
The general trend is one of increasing coordination number with pressure, culminating in a hexagonal close-packed allotrope at about 40 gigapascals known as Si–VII (the standard modification being Si–I).
31Si may be produced by the neutron activation of natural silicon and is thus useful for quantitative analysis; it can be easily detected by its characteristic beta decay to stable 31P, in which the emitted electron carries up to 1.48 MeV of energy.
Between 950 °C and 1160 °C, the formation rate of the vitreous dioxide rapidly increases, and when 1400 °C is reached, atmospheric nitrogen also reacts to give the nitrides SiN and Si3N4.
This oxide layer nevertheless does not prevent reaction with the halogens; fluorine attacks silicon vigorously at room temperature, chlorine does so at about 300 °C, and bromine and iodine at about 500 °C.
[64] Silicon is the eighth most abundant element in the universe, coming after hydrogen, helium, carbon, nitrogen, oxygen, iron, and neon.
This sequence shows a trend toward increasingly complex silicate units with cooling, and the introduction of hydroxide and fluoride anions in addition to oxides.
These compounds are volatile and hence can be purified by repeated fractional distillation, followed by reduction to elemental silicon with very pure zinc metal as the reducing agent.
The spongy pieces of silicon thus produced are melted and then grown to form cylindrical single crystals, before being purified by zone refining.
[78][79] Most elemental silicon produced remains as a ferrosilicon alloy, and only approximately 20% is refined to metallurgical grade purity (a total of 1.3–1.5 million metric tons/year).
In practice, pure silicon is doped with small concentrations of certain other elements, which greatly increase its conductivity and adjust its electrical response by controlling the number and charge (positive or negative) of activated carriers.
Silicon has become the most popular material for both high power semiconductors and integrated circuits because it can withstand the highest temperatures and greatest electrical activity without suffering avalanche breakdown (an electron avalanche is created when heat produces free electrons and holes, which in turn pass more current, which produces more heat).
In addition, the insulating oxide of silicon is not soluble in water, which gives it an advantage over germanium (an element with similar properties which can also be used in semiconductor devices) in certain fabrication techniques.
[79] Silicon quantum dots are created through the thermal processing of hydrogen silsesquioxane into nanocrystals ranging from a few nanometers to a few microns, displaying size dependent luminescent properties.
The effect can also be achieved in reverse with a donor molecule having its highest occupied molecular orbital (HOMO) slightly higher than a valence band edge of the quantum dot, allowing electrons to transfer between them, filling the holes and preventing recombination.
This has been shown to improve cell wall strength and structural integrity in some plants, thereby reducing insect herbivory and pathogenic infections.
[98][99][96] Several horticultural crops are known to protect themselves against fungal plant pathogens with silica, to such a degree that fungicide application may fail unless accompanied by sufficient silicon nutrition.
Silicon is considered an alternative to carbon, as it can create complex and stable molecules with four covalent bonds, required for a DNA-analog, and it is available in large quantities.