Transgenerational epigenetic inheritance
[5] There are several mechanisms of TEI that have shown to affect germline reprogramming, such as transgenerational increases in susceptibility to diseases, mutations, and stress inheritance.
[8] The complex cell signaling pathways of multicellular organisms such as plants and humans can make understanding the mechanisms of this inherited process very difficult.
Currently, self-sustaining feedback loops, spatial templating, chromatin marking, and RNA-mediated pathways modify epigenes of individual cells.
Due to the multivariate nature of environmental factors, it is difficult for researchers to pinpoint the exact cause of epigenetic changes outside of a laboratory setting.
[39][47] The lack of darkly pigmented individuals in the F2 progeny is an example of non-Mendelian inheritance and further research has suggested that the B-I allele is converted to B' via epigenetic mechanisms.
Genetically identical reciprocal F1 hybrid triploids have been shown to display transgenerational epigenetic effects on viable F2 seed development.
[53] It is difficult to trace TEI in animals due to the reprogramming of genes during meiosis and embryogenesis, especially in wild populations that are not reared in a lab setting.
[citation needed] An example of how the environment within the womb can affect the health of an offspring is the Dutch hunger winter of 1944–45 and its causal effect on induced transgenerational epigenetic inherited diseases.
In all 19 informative cases, the epimutations that, together with physiological imprinting and therefore silencing of the other allele, were causing these syndromes were localized on a chromosome with a specific parental and grandparental origin.
[59] Furthermore, evidence collected in various studies utilizing model systems (i.e. animals) have found that exposure during parental generations can result in multigenerational and transgenerational inheritance of breast cancer.
[59] More recently, studies have discovered a connection between the adaptation of male germinal cells via pre-conception paternal diets and the regulation of breast cancer in developing offspring.
[59] More specifically, studies have begun to uncover new data that underscores a relationship between transgenerational epigenetic inheritance of breast cancer and ancestral alimentary components or associated markers, such as birth weight.
[61] These maternal risk factors and environmental stressors coupled with transgenerational epigenetic changes can result in prolonged insult to the signaling pathways associated with the vascular development during fetal stages, thus increasing the likelihood of having PAH.
[65][non-primary source needed] Thus, children with methylated glucocorticoid-receptor genes experience an altered response to stress, ultimately leading to a higher susceptibility of experiencing anxiety.
[72] Experimentally demethylated seeds of the model organism Arabidopsis thaliana have significantly higher mortality, stunted growth, delayed flowering, and lower fruit set,[73] indicating that epigenes may increase fitness.
[78] The progeny of mice survived the Candida albicans infection via functional, transcriptional, and epigenetic changes linked to the immune gene loci.
[79] Double-mating experiments with the red flour beetle demonstrated that paternal transgenerational immune priming is mediated by sperm or seminal fluid which enhances survival upon exposure to pathogens and contribute to epigenetic changes.
[82] In another case, it was suggested that endocrine disruption had a feedback loop interaction with methylation of varying genomic sites in Menidia beryllina, which may have been a function of TEI.
[85] The early differentiation of animal germlines is likely to preclude epigenetic marking occurring later in development, while in plants and fungi somatic cells may be incorporated into the germ line.
[19] As of late, most of the experimental models utilizing mice and limited observations in humans have only found epigenetically inherited traits that are detrimental to the health of both organisms.
This insight led to the practical application of selective breeding of plants and animals, but did not address the central question of inheritance: how are these traits conserved between generations, and what causes variation?
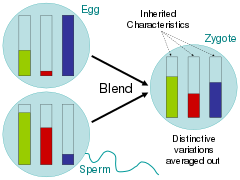
[citation needed] Addressing these related questions, scientists during the time of the Enlightenment largely argued for the blending hypothesis, in which parental traits were homogenized in the offspring much like buckets of different colored paint being mixed together.
[88] Critics of Charles Darwin's On the Origin of Species, pointed out that under this scheme of inheritance, variation would quickly be swamped by the majority phenotype.
[citation needed] Unknown to most of the European scientific community, the monk Gregor Mendel had resolved the question of how traits are conserved between generations through breeding experiments with pea plants.
[90] Charles Darwin thus did not know of Mendel's proposed "particulate inheritance" in which traits were not blended but passed to offspring in discrete units that we now call genes.
[citation needed] In his 1809 book, Philosophie Zoologique,[91] Jean-Baptiste Lamarck recognized that each species experiences a unique set of challenges due to its form and environment.
[citation needed] Lamarckism, as this body of thought became known, was the standard explanation for change in species over time when Charles Darwin and Alfred Russel Wallace co-proposed a theory of evolution by natural selection in 1859.
[95] Researchers discussed Waddington's epigenetics sporadically - it became more of a catch-all for puzzling non-genetic heritable characters rather than a concept advancing the body of inquiry.
Outlining the central dogma of molecular biology, Francis Crick[98] succinctly stated, "DNA is held in a configuration by histone[s] so that it can act as a passive template for the simultaneous synthesis of RNA and protein[s].
[citation needed] There has been critical discussion of mainstream evolutionary theory by Edward J Steele, Robyn A Lindley and colleagues,[101][102][103][104][105] Fred Hoyle and N. Chandra Wickramasinghe,[106][107][108] Yongsheng Liu[109][110] Denis Noble,[111][112] John Mattick[113] and others that the logical inconsistencies as well as Lamarckian Inheritance effects involving direct DNA modifications, as well as the just described indirect, viz.