Glossary of chemistry terms
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment.
Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
Note: All periodic table references refer to the IUPAC Style of the Periodic Table.
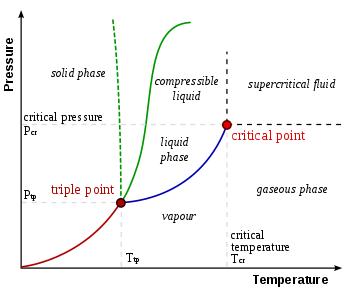
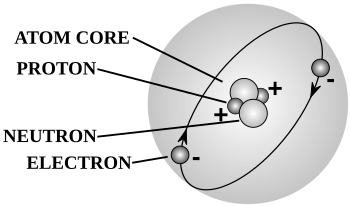
Also acid ionization constant or acidity constant.Also actinoids.Also paraffin.Also olefin.Also acetylene.Also enplethy, chemical amount, or simply amount.Also amphiprotic.Also proton number.Also kindling point.Also main chain.Also Rutherford–Bohr model.Also ebullition.Also Florence flask.Also vaporization point.Also simply called a buffer.Also stopper or cork.Also spelled buret.Also simply CAS Number.Also simply called a chemical.Also pure substance or simply substance.Also chromometer.Also molecular bond.Also unified atomic mass unit (u).Also drying agent.Also hydrogen-2 or heavy hydrogen, and symbolized 2H or D.Also coordinate covalent bond, coordinate bond, dative bond, and semipolar bond.Also solvation.Also malleability.Also electron magnetic moment.Also crystallization point.Also depression of freezing point.Also family.Also simply called Hess' law.Informally synonymous with proton.Also universal gas constant.Also general gas equation.Also ketoacid.Also lanthanoids.Also referred to as visible light.Also atomic mass number or nucleon number.Also liquefaction point.Also carbinyl.Also molality.Also molarity, amount concentration, or substance concentration.Also mole fraction.Sometimes used interchangeably with molecular weight and formula weight.Also inert gas.Also Lewis octet rule.Also orbital hybridization.Also osmolarity.Also oxidation number.Also oxidant, oxidizer, or electron acceptor.Also oxyacid or oxacid.Also amyl.Also simply the periodic table.Also peroxide and sometimes peroxo.Also spelled pipet.Also protogenic.(pl.)
quantaAlso free radical.Also radioisotope.Also called rare-earth metals or used interchangeably with lanthanides.Also rate law.Also rate-limiting step.Sometimes used interchangeably with reagent.Also simply intermediate.Also activity series.Also reductant, reducer, or electron donor.Also ultrasonication.Also massic heat capacity.Also stereocenter.Also spatial isomer.Also constitutional isomer.Also titrimetry or volumetric analysis.Also superheavy elements.Also transuranium elements.Also Dewar flask or thermos.Also equilibrium vapor pressure.Also boiling.Also water of hydration.Also bench chemistry or classical chemistry.Also inner salt and dipolar ion.





2 (right), is formed by a covalent bond when two single hydrogen atoms share two electrons between them.