Fatty acid
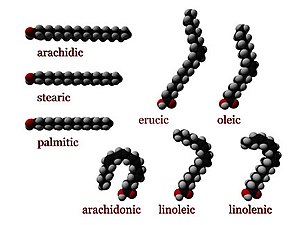
Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28.
[6] Fatty acids are classified in many ways: by length, by saturation vs unsaturation, by even vs odd carbon content, and by linear vs branched.
Some trans fatty acids also occur naturally in the milk and meat of ruminants (such as cattle and sheep).
Most fatty acids are even-chained, e.g. stearic (C18) and oleic (C18), meaning they are composed of an even number of carbon atoms.
Most common fatty acids are straight-chain compounds, with no additional carbon atoms bonded as side groups to the main hydrocarbon chain.
The position of each carbon atom in the backbone of a fatty acid is usually indicated by counting from 1 at the −COOH end.
Although fatty acids can be of diverse lengths, in this second convention the last carbon in the chain is always labelled as ω (omega), which is the last letter in the Greek alphabet.
[d] In either numbering scheme, the position of a double bond in a fatty acid chain is always specified by giving the label of the carbon closest to the carboxyl end.
In the context of human diet and fat metabolism, unsaturated fatty acids are often classified by the position of the double bond closest between to the ω carbon (only), even in the case of multiple double bonds such as the essential fatty acids.
[15] FFAs also form from triglyceride food oils and fats by hydrolysis, contributing to the characteristic rancid odor.
[17] In animals, fatty acids are formed from carbohydrates predominantly in the liver, adipose tissue, and the mammary glands during lactation.
However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids occurs.
[19][20] Malonyl-CoA is then involved in a repeating series of reactions that lengthens the growing fatty acid chain by two carbons at a time.
The levels of "free fatty acids" in the blood are limited by the availability of albumin binding sites.
This maintenance cost has been argued to be one of the key causes for the high metabolic rates and concomitant warm-bloodedness of mammals and birds.
[26][27] The following table gives the fatty acid, vitamin E and cholesterol composition of some common dietary fats.
Typically, transesterification is practiced in the conversion of fats to fatty acid methyl esters.
This analysis is used to determine the free fatty acid content of fats; i.e., the proportion of the triglycerides that have been hydrolyzed.
Neutralization of fatty acids, like saponification, is a widely practiced route to metallic soaps.
Since the saturated fatty acids are higher melting than the unsaturated precursors, the process is called hardening.
It is transported via the lymphatic system and the thoracic duct up to a location near the heart (where the arteries and veins are larger).
In the final step (oxidative phosphorylation), reactions with oxygen release a lot of energy, captured in the form of large quantities of ATP.
[38] The stratum corneum – the outermost layer of the epidermis – is composed of terminally differentiated and enucleated corneocytes within a lipid matrix.
[39] Together with cholesterol and ceramides, free fatty acids form a water-impermeable barrier that prevents evaporative water loss.
[39] Generally, the epidermal lipid matrix is composed of an equimolar mixture of ceramides (about 50% by weight), cholesterol (25%), and free fatty acids (15%).
[39][40] The relative abundance of the different fatty acids in the epidermis is dependent on the body site the skin is covering.
[40] There are also characteristic epidermal fatty acid alterations that occur in psoriasis, atopic dermatitis, and other inflammatory conditions.
[39][40] The chemical analysis of fatty acids in lipids typically begins with an interesterification step that breaks down their original esters (triglycerides, waxes, phospholipids etc.)
[42] Other separation techniques include high-performance liquid chromatography (with short columns packed with silica gel with bonded phenylsulfonic acid groups whose hydrogen atoms have been exchanged for silver ions).