List of fentanyl analogues
This is a list of fentanyl analogues (sometimes referred to as Fentalogs),[1][2][3] including both compounds developed by pharmaceutical companies for legitimate medical use, and those which have been sold as designer drugs and reported to national drug control agencies such as the DEA, or transnational agencies such as the EMCDDA and UNODC.
[4][5][6][7][8][9][10] This is not a comprehensive listing of fentanyl analogues, as more than 1400 compounds from this family have been described in the scientific and patent literature,[11][12][13][14][15] but it includes many notable compounds that have reached late-stage human clinical trials, or which have been identified as having been sold as designer drugs, as well as representative examples of significant structural variations reported in the scientific and patent literature.
The structural variations among fentanyl-related substances can impart profound pharmacological differences between these drugs, especially with respect to potency and efficacy.
[21] Temporary control of fentanyl-related substances in Schedule I was extended through December 31, 2024 by Public Law 117-328.
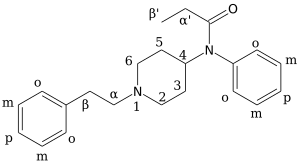
This section is written to help illustrate the basic ring structure of fentanyl and what popular analogues reference on the carbon skeleton, helping a chemist working with fentanyl analogues quickly and consistently navigate the nomenclature system.
Depending on the organic acid used in the amide condensation, different analogues of fentanyl will be produced.
Many analogues of great potency, such as ohmfentanyl and lofentanyl possess methyl acetate groups added to the 4-carbon (of the piperidine ring, in the para- position relative to the annular nitrogen).
These analogues can possess a wide variety of modified pharmacological properties, including increased and decreased potency (receptor binding efficiency), increased or decreased half-life (metabolic binding efficiency) or other side effects on human physiology.
Other substituents such as hydroxy, chloro, fluoro, and a wide variety of alkyl groups, are also substituted in place of these methylations to produce psychoactive analogues of fentanyl, but because they often use the same skeletal naming conventions as the simple methyl analogues, we did not reproduce them all in the image here.
The first modifications is the removal of the phenthyl moeity from the piperidinyl nitrogen, depicted here as hydrolysis yielding phenethanol.
This leaves only 2 R/S assignments that follow the orientation of the stereocenter at the C-α (alpha carbon) position for the real α-methylfentanyl.
This uses a similar structure to analogize the three potential stereocenters in ohmfentanyl, namely the 4-C, the 3-C, and the β-C (beta carbon).
These fundamentals are typically enough to help a chemists navigate the world of fentanyl analogues proficiently.
However, the presence of three six-membered rings which can each be independently substituted can easily lead to confusion, especially with the inconsistent use of prime notation.
This means that two positional isomers with the same molecular weight, which may be difficult to tell apart without detailed chemical analysis, may be hundreds or even thousands of times different in pharmacological potency.