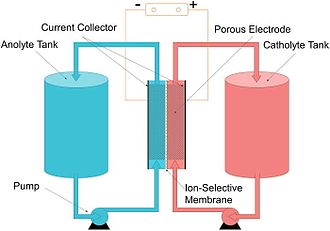
Flow battery
[2][3] Ion transfer inside the cell (accompanied by current flow through an external circuit) occurs across the membrane while the liquids circulate in their respective spaces.
Flow batteries have certain technical advantages over conventional rechargeable batteries with solid electroactive materials, such as independent scaling of power (determined by the size of the stack) and of energy (determined by the size of the tanks), long cycle and calendar life,[6] and potentially lower total cost of ownership,.
The heavier weight results mostly from the need to use a solvent (usually water) to maintain the redox active species in the liquid phase.
[citation needed] Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.
[9] Walther Kangro, an Estonian chemist working in Germany in the 1950s, was the first to demonstrate flow batteries based on dissolved transition metal ions: Ti–Fe and Cr–Fe.
Mixed solutions (i.e. comprising both chromium and iron species in the negolyte and in the posolyte) were used in order to reduce the effect of time-varying concentration during cycling.
[13] In 2022, Dalian, China began operating a 400 MWh, 100 MW vanadium flow battery, then the largest of its type.
Hokkaido’s flow battery farm was the biggest in the world when it opened in April 2022 — until China deployed one eight times larger that can match the output of a natural gas plant.
[26] Because they employ heterogeneous electron transfer rather than solid-state diffusion or intercalation they are more similar to fuel cells than to conventional batteries.
Cr–Fe chemistry has disadvantages, including hydrate isomerism (i.e. the equilibrium between electrochemically active Cr3+ chloro-complexes and inactive hexa-aqua complex and hydrogen evolution on the negode.
This chemistry's advantages include four oxidation states within the electrochemical voltage window of the graphite-aqueous acid interface, and thus the elimination of the mixing dilution, detrimental in Cr–Fe RFBs.
This extends the life of the low-cost carbon electrodes and reduces the impact of side reactions, such as H2 and O2 evolutions, resulting in many year durability and many cycle (15,000–20,000) lives, which in turn results in a record low levelized cost of energy (LCOE, system cost divided by usable energy, cycle life, and round-trip efficiency).
These long lifetimes allow for the amortization of their relatively high capital cost (driven by vanadium, carbon felts, bipolar plates, and membranes).
Weng et al. reported a vanadium–metal hydride hybrid flow battery with an experimental OCV of 1.93 V and operating voltage of 1.70 V, relatively high values.
Preliminary data using a fluctuating simulated power input tested the viability toward kWh scale storage.
During charging, PFB combines hydrogen ions produced from splitting water with electrons and metal particles in one electrode of a fuel cell.
As of 2021, organic RFB experienced low durability (i.e. calendar or cycle life, or both) and have not been demonstrated on a commercial scale.
In larger-scale energy storage, lower solvent cost and higher conductivity give AORFBs greater commercial potential, as well as offering the safety advantages of water-based electrolytes.
[46] AORFBs used methyl viologen as an anolyte and 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl as a catholyte at pH neutral conditions, plus NaCL and a low-cost anion exchange membrane.
[47][48] Neutral AORFBs can be more environmentally friendly than acid or alkaline alternatives, while showing electrochemical performance comparable to corrosive RFBs.
Quinones accept two units of electrical charge, compared with one in conventional catholyte, implying twice as much energy in a given volume.
Replacing hydrobromic acid with a less toxic alkaline solution (1 M KOH) and ferrocyanide[56] was less corrosive, allowing the use of inexpensive polymer tanks.
In 2021 a reversible ketone (de)hydrogenation demonstration cell operated continuously for 120 days over 1,111 charging cycles at room temperature without a catalyst, retaining 97% percent capacity.
The ligands can be chelates such as EDTA, and can enable the electrolyte to be in neutral or alkaline conditions under which metal aquo complexes would otherwise precipitate.
By blocking the coordination of water to the metal, organic ligands can inhibit metal-catalyzed water-splitting reactions, resulting in higher voltage aqueous systems.
A membraneless battery[71] relies on laminar flow in which two liquids are pumped through a channel, where they undergo electrochemical reactions to store or release energy.
The battery was based on immiscible organic catholyte and aqueous anolyte liquids, which exhibited high capacity retention and Coulombic efficiency during cycling.
[74] A lithium–sulfur system arranged in a network of nanoparticles eliminates the requirement that charge moves in and out of particles that are in direct contact with a conducting plate.
[80] In 2022, Influit Energy announced a flow battery electrolyte consisting of a metal oxide suspended in an aqueous solution.
Flow batteries are normally considered for relatively large (1 kWh – 10 MWh) stationary applications with multi-hour charge-discharge cycles.