Flutamide
Flutamide, sold under the brand name Eulexin among others, is a nonsteroidal antiandrogen (NSAA) which is used primarily to treat prostate cancer.
[12] Side effects in men include breast tenderness and enlargement, feminization, sexual dysfunction, and hot flashes.
The medication has largely been replaced by newer and improved NSAAs, namely bicalutamide and enzalutamide, due to their better efficacy, tolerability, safety, and dosing frequency (once per day), and is now relatively little-used.
[citation needed] DHT, and to a significantly smaller extent, testosterone, stimulate prostate cancer cells to grow.
[citation needed] There have been studies to investigate the benefit of adding an antiandrogen to surgical orchiectomy or its continued use with a GnRH analogue (combined androgen blockade (CAB)).
[citation needed] Unfortunately, therapies which lower testosterone levels, such as orchiectomy or GnRH analogue administration, also have significant side effects.
[21][22] The medication, at a dosage of 750 mg/day (250 mg three times daily), has also been found to be equivalent in effectiveness to 250 mg/day oral cyproterone acetate as a monotherapy in the treatment of prostate cancer in a large-scale clinical trial of 310 patients, though its side effect and toxicity profiles (including gynecomastia, diarrhea, nausea, loss of appetite, and liver disturbances) were regarded as considerably worse than those of cyproterone acetate.
[23] A dosage of 750 mg/day flutamide (250 mg/three times a day) is roughly equivalent in terms of effectiveness to 50 mg/day bicalutamide when used as the antiandrogen component in combined androgen blockade in the treatment of advanced prostate cancer.
[24] Flutamide has been used to prevent the effects of the testosterone flare at the start of GnRH agonist therapy in men with prostate cancer.
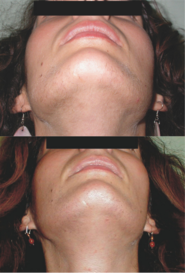
[33] Flutamide has been researched and used extensively in the treatment of androgen-dependent skin and hair conditions in women including acne, seborrhea, hirsutism, and scalp hair loss, as well as in hyperandrogenism (e.g., in polycystic ovary syndrome or congenital adrenal hyperplasia), and is effective in improving the symptoms of these conditions.
Although flutamide continues to be used for these indications, its use in recent years has been limited due to the risk of potentially fatal hepatotoxicity, and it is no longer recommended as a first- or second-line therapy.
[52][34] The medication shows equivalent or superior effectiveness to other antiandrogens including spironolactone, cyproterone acetate, and finasteride in the treatment of hirsutism, although its relatively high risk of hepatotoxicity makes it unfavorable compared to these other options.
[60] In a comparative study, flutamide significantly improved scalp hair growth (21% reduction in Ludwig scores) in hyperandrogenic women after 1 year of treatment, whereas cyproterone acetate and finasteride were ineffective.
[58][61] Flutamide has been used in case reports to decrease the frequency of spontaneous orgasms, for instance in men with post-orgasmic illness syndrome.
In men, a variety of side effects related to androgen deprivation may occur, the most common being gynecomastia and breast tenderness.
[44] The only common side effect of flutamide in women is dry skin (75%), which can be attributed to a reduction of androgen-mediated sebum production.
[74][75][69] Tamoxifen, a selective estrogen receptor modulator (SERM) with predominantly antiestrogenic actions, can counteract flutamide-induced gynecomastia and breast pain in men.
[41] In a comparative trial of combined androgen blockade for prostate cancer, the rate of diarrhea was 26% for flutamide and 12% for bicalutamide.
[78][79] A 2021 review of the literature found 15 cases of serious hepatotoxicity in women treated with flutamide, including 7 liver transplantations and 2 deaths.
[90][91][92] Specifically, flutamide and particularly its major metabolite hydroxyflutamide inhibit enzymes in the mitochondrial electron transport chain in hepatocytes, including respiratory complexes I (NADH ubiquinone oxidoreductase), II (succinate dehydrogenase), and V (ATP synthase), and thereby reduce cellular respiration via ATP depletion and hence decrease cell survival.
[90][93] In contrast to flutamide and hydroxyflutamide, which severely compromise hepatocyte cellular respiration in vitro, bicalutamide does not significantly do so at the same concentrations and is regarded as non-mitotoxic.
[13] In accordance, the combination of paracetamol (acetaminophen) and flutamide appears to result in additive to synergistic hepatotoxicity, indicating a potential drug interaction.
[15] The incidence of interstitial pneumonitis with flutamide was found to be 0.04% (4 per 10,000) in a large clinical cohort of 41,700 prostate cancer patients.
[3][109] However, it can have some indirect estrogenic effects via increased levels of estradiol secondary to AR blockade, and this involved in the gynecomastia it can produce.
[112] In accordance, 50 mg/day bicalutamide has been found to possess equivalent or superior effectiveness to 750 mg/day flutamide in a large clinical trial for prostate cancer.
[122] In accordance, flutamide has been found to slightly but significantly lower androgen levels in GnRH analogue-treated male prostate cancer patients[123] and women with polycystic ovary syndrome.
[107] However, the clinical significance of this action may be limited when flutamide is given without a GnRH analogue to non-castrated men, as the medication markedly elevates testosterone levels into the high normal male range via prevention of AR activation-mediated negative feedback on the hypothalamic–pituitary–gonadal axis in this context.
[143][144] Flutamide was not introduced in the United States until 1989; it was specifically approved by the U.S. Food and Drug Administration for the treatment of metastatic prostate cancer in combination with a gonadotropin-releasing hormone (GnRH) analogue.
[158] Flutamide was studied for the treatment of advanced breast cancer in two phase II clinical trials but was found to be ineffective.
[164][165][166][167] Flutamide was found to be effective in the treatment of obsessive–compulsive disorder (OCD) in men with comorbid Tourette's syndrome in one small randomized controlled trial.