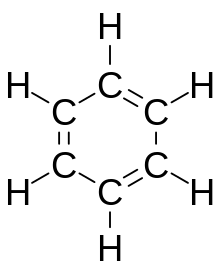
Aromatic compound
"[1] The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood.
Aromatic compounds have the following general properties: Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives.
Aromatic compounds are commonly used in organic synthesis and are involved in many reaction types, following both additions and removals, as well as saturation and dearomatization.
Heteroarenes are aromatic compounds, where at least one methine or vinylene (-C= or -CH=CH-) group is replaced by a heteroatom: oxygen, nitrogen, or sulfur.
Key industrial aromatic hydrocarbons are benzene, toluene, xylene called BTX.
[6] Its bonding nature was first recognized independently by Joseph Loschmidt and August Kekulé in the 19th century.
[5] The circle symbol for aromaticity was introduced by Sir Robert Robinson and his student James Armit in 1925 and popularized starting in 1959 by the Morrison & Boyd textbook on organic chemistry.
Some argue that, in order to stay in line with Robinson's originally intended proposal, the use of the circle symbol should be limited to monocyclic 6 π-electron systems.
When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ortho, meta, and para are devised.
[10] Given that both the methyl and hydroxyl group are ortho-para directors, the ortho and para isomers are typically favoured.
[11] Another example of a non-benzylic monocyclic arene is the cyclopropenyl (cyclopropenium cation), which satisfies Hückel's rule with an n equal to 0.
[25] The compound resorcinol, hydrogenated with Raney nickel in presence of aqueous sodium hydroxide forms an enolate which is alkylated with methyl iodide to 2-methyl-1,3-cyclohexandione:[26] In dearomatization reactions the aromaticity of the reactant is lost.