Influenza vaccine
During the worldwide Spanish flu pandemic of 1918, "Pharmacists tried everything they knew, everything they had ever heard of, from the ancient art of bleeding patients, to administering oxygen, to developing new vaccines and serums (chiefly against what we call Hemophilus influenzae – a name derived from the fact that it was originally considered the etiological agent – and several types of pneumococci).
Meanwhile, maximum military containment efforts succeeded unexpectedly in confining the new strain to the single army base where it had originated.
[97] Effectiveness against hospitalized influenza illness in the 2019–2020 United States flu season was 41% overall and 54% in people aged 65 years or older.
[104] According to a 2016 study by faculty at the University of New South Wales, getting a flu shot was as effective or better at preventing a heart attack than even quitting smoking.
[107] In April 2002, the Advisory Committee on Immunization Practices (ACIP) encouraged that children 6 to 23 months of age be vaccinated annually against influenza.
[137][138][139] Per Advisory Committee on Immunization Practices guidelines, Fluad can be used as an alternative to other influenza vaccines approved for people 65 years and older.
[135] Vaccinating healthcare workers who work with elderly people is recommended in many countries, with the goal of reducing influenza outbreaks in this vulnerable population.
[145][146] As well as protecting mother and child from the effects of an influenza infection, the immunization of pregnant women tends to increase their chances of experiencing a successful full-term pregnancy.
[95] While side effects of the flu vaccine may occur, they are usually minor, including soreness, redness, swelling around the point of injection, headache, fever, nausea, or fatigue.
[149] Side effects of a nasal spray vaccine may include runny nose, wheezing, sore throat, cough, or vomiting.
[159] Studies examining the safety of influenza vaccines in people with severe egg allergies found that anaphylaxis was very rare, occurring in 1.3 cases per million doses given.
[207] Furthermore, the CDC recommends that healthcare personnel who care for severely immunocompromised persons receive injections (TIV or QIV) rather than LAIV.
[181] During the 2009 pandemic, low uptake by healthcare workers was seen in countries including the UK,[181] Italy,[217] Greece,[218] and Hong Kong.
[220][221][222] The main reason to vaccinate health care workers is to prevent staff from spreading flu to their patients and to reduce staff absence at a time of high service demand, but the reasons health care workers state for their decisions to accept or decline vaccination may more often be to do with perceived personal benefits.
[231][225] In the Northern hemisphere, the manufacturing process begins following the announcement (typically in February) of the WHO recommended strains for the winter flu season.
This technique is expected to be more scalable and avoid problems with eggs, such as allergic reactions and incompatibility with strains that affect avians like chickens.
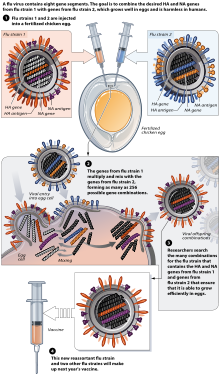
[235] A second hole is made at the top of the egg, where the influenza virus is injected in the allantoic cavity, past the chorioallantoic membrane.
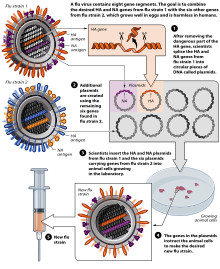
[237] Methods of vaccine generation that bypass the need for eggs include the construction of influenza virus-like particles (VLP).
VLP resemble viruses, but there is no need for inactivation, as they do not include viral coding elements, but merely present antigens in a similar manner to a virion.
There is evidence that some VLPs elicit antibodies that recognize a broader panel of antigenically distinct viral isolates compared to other vaccines in the hemagglutination-inhibition assay (HIA).
[159] According to the WHO, as of 2019[update], countries where influenza vaccine is produced include:[249] In addition, Kazakhstan, Serbia, and Thailand had facilities in the final stages of establishing production.
[252] The uncertainty in influenza cost-effectiveness models can partially be explained by the complexities involved in estimating the disease burden,[253] as well as the seasonal variability in the circulating strains and the match of the vaccine.
[254][255] In healthy working adults (aged 18–49 years), a 2012 review found that vaccination was generally not cost-saving, with the suitability for funding being dependent on the willingness to pay to obtain the associated health benefits.
Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains, and induce an immune response.
More specifically, researchers have used data from Twitter and Microsoft's Bing search engine and proposed a statistical framework that, after a series of operations, maps this information to estimates of the influenza-like illness reduction percentage in areas where vaccinations have been performed.
Audenz is approved for use in persons six months of age and older at increased risk of exposure to the influenza A virus H5N1 subtype contained in the vaccine.
[268][270] The challenges for researchers are to identify single antibodies that could neutralize many subtypes of the virus so that they could be useful in any season, and that target conserved domains that are resistant to antigenic drift.
A meta-analysis of 9001 randomized trial participants found that influenza vaccination was associated with a 34% lower risk of major adverse cardiovasular events when compared to placebo.
It is generally recognized that in many cases such schedules may not maintain protective levels of antibody and more frequent administration is advised in high-risk situations.
Standard commercial swine flu vaccines are effective in controlling the problem when the virus strains match enough to have significant cross-protection.