Folliculogenesis
In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte.
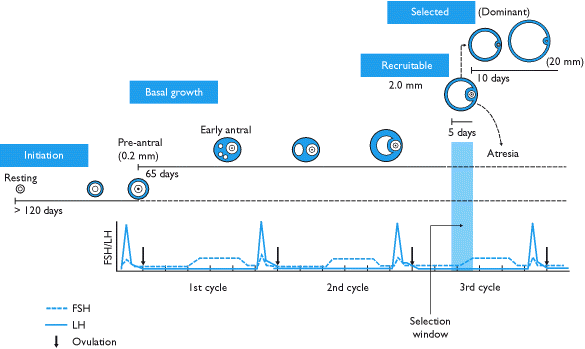
From the whole pool of follicles a woman is born with, only 0.1% of them will rise ovulation, whereas 99.9% will break down (in a process called follicular atresia).
[citation needed] During follicular development, primordial follicles undergo a series of critical changes in character, both histologically and hormonally.
At this stage in development, they become dependent on hormones, particularly FSH which causes a substantial increase in their growth rate.
It has generally been documented to occur once in the early- to mid- follicular phase of the menstrual cycle, leading to ovulation.
A few develop fully to produce a secondary oocyte which is released by rupture of the follicle in a process called ovulation.
Because primordial follicles can be dormant for up to 50 years in humans, the length of the ovarian cycle does not include this time.
Research has shown that initial recruitment is mediated by the counterbalance of various stimulatory and inhibitory hormones and locally produced growth factors.
An intricate network of capillary vessels forms between these two thecal layers and begins to circulate blood to and from the follicle.
The late-term secondary follicle is marked histologically and structurally by a fully grown oocyte surrounded by a zona pellucida, approximately nine layers of granulosa cells, a basal lamina, a theca interna, a capillary net, and a theca externa.
Granulosa and theca cells continue to undergo mitotis concomitant with an increase in antrum volume.
Under action of an oocyte-secreted morphogenic gradient, the granulosa cells of the tertiary follicle undergo differentiation into four distinct subtypes: corona radiata, surrounding the zona pellucida; membrana, interior to the basal lamina; periantral, adjacent to the antrum and cumulus oophorus, which connects the membrana and corona radiata granulosa cells together.
This process of follicle death is known as atresia, and it is characterized by radical apoptosis of all constituent cells and the oocyte.
[8] Performing controlled ovarian hyperstimulation leads to a greater recruitment of follicles, growing at about 1.6 mm per day.
The oocyte will now travel down one of the fallopian tubes to eventually be discharged through menstruation in the case that it is unfertilized or if it is not successfully implanted in the uterus (if previously fertilized).
This peak (through AMPc) activates the pro-inflammatory genes, which cause the break of the follicle wall and the oocyte gets out.
Estrogen has since dropped to negative stimulatory levels after ovulation and therefore serves to maintain the concentration of FSH and LH.
However, coordinated enzyme signalling and the time-specific expression of hormonal receptors ensures that follicle growth does not become disregulated during these premature spikes.
Recently, two publications have challenged the idea that a finite number of follicles are set around the time of birth.
[11][12] Renewal of ovarian follicles from germline stem cells (originating from bone marrow and peripheral blood) was reported in the postnatal mouse ovary.
[4] In 2010, researchers at the University of Edinburgh determined that by the time women are 30 years old, only 10% of their non-growing follicles (NGFs) remain.

1 - Menstruation
2 - Developing follicle
3 - Mature follicle
4 - Ovulation
5 - Corpus luteum
6 - Deterioration of corpus luteum