Frederick Soddy
It needed careful work by Soddy and Rutherford to prove that atomic transmutation was in fact occurring.
[10] In 1903, with Sir William Ramsay at University College London, Soddy showed that the decay of radium produced helium gas.
After leaving the experiment running for a long period of time, a spectral analysis of the contents of the former evacuated space revealed the presence of helium.
The work that Soddy and his research assistant Ada Hitchins did at Glasgow and Aberdeen showed that uranium decays to radium.
In 1918, working with the Scottish scientist John Arnold Cranston, he announced the discovery of an isotope of the element later named protactinium.
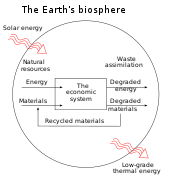
Organizations: In four books written from 1921 to 1934, Soddy carried on a "campaign for a radical restructuring of global monetary relationships",[21] offering a perspective on economics rooted in physics – the laws of thermodynamics, in particular – and was "roundly dismissed as a crank".
[21] While most of his proposals – "to abandon the gold standard, let international exchange rates float, use federal surpluses and deficits as macroeconomic policy tools that could counter cyclical trends, and establish bureaus of economic statistics (including a consumer price index) in order to facilitate this effort" – are now conventional practice, his critique of fractional-reserve banking still "remains outside the bounds of conventional wisdom" although a recent paper by the IMF reinvigorated his proposals.
[21][22] Soddy wrote that financial debts grew exponentially at compound interest but the real economy was based on exhaustible stocks of fossil fuels.
[26] The influence of his writing can be gauged, for example, in this quote from Ezra Pound: Professor Frederick Soddy states that the Gold Standard monetary system has wrecked a scientific age!
The world's bankers ... have not been content to take their share of modern wealth production – great as it has been – but they have refused to allow the masses of mankind to receive theirs.
[29] He received the Nobel Prize in Chemistry in 1921 and the same year was elected member of the International Atomic Weights Committee.