Gallium(III) chloride
Gallium(III) chloride is an inorganic chemical compound with the formula GaCl3 which forms a monohydrate, GaCl3·H2O.
As a consequence of its molecular nature and associated low lattice energy, gallium(III) chloride has a lower melting point vs the aluminium and indium trihalides.
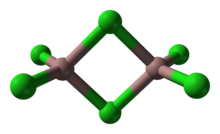
[8] Gallium(III) chloride is a diamagnetic and deliquescent colorless white solid that melts at 77.9 °C and boils at 201 °C without decomposition to the elements.
This low melting point results from the fact that it forms discrete Ga2Cl6 molecules in the solid state.
[14] In a molten mixture of KCl and GaCl3, the following equilibrium exists: When dissolved in water, gallium(III) chloride dissociates into the octahederal [Ga(H2O)6]3+ and Cl− ions forming an acidic solution, due to the hydrolysis of the hexaaquogallium(III) ion:[15] In basic solution, it hydrolyzes to gallium(III) hydroxide, which redissolves with the addition of more hydroxide, possibly to form Ga(OH)4−.
[7] 110 tons of gallium(III) chloride aqueous solution was used in the GALLEX and GNO experiments performed at Laboratori Nazionali del Gran Sasso in Italy to detect solar neutrinos.
In these experiments, germanium-71 was produced by neutrino interactions with the isotope gallium-71 (which has a natural abundance of 40%), and the subsequent beta decays of germanium-71 were measured.