Germanium
Presently, the major end uses are fibre-optic systems, infrared optics, solar cell applications, and light-emitting diodes (LEDs).
[14][15][23] He also determined an atomic weight of 72.32 by analyzing pure germanium tetrachloride (GeCl4), while Lecoq de Boisbaudran deduced 72.3 by a comparison of the lines in the spark spectrum of the element.
[24] Winkler was able to prepare several new compounds of germanium, including fluorides, chlorides, sulfides, dioxide, and tetraethylgermane (Ge(C2H5)4), the first organogermane.
Silicon has superior electrical properties, but it requires much greater purity that could not be commercially achieved in the early years of semiconductor electronics.
[33] Meanwhile, the demand for germanium for fiber optic communication networks, infrared night vision systems, and polymerization catalysts increased dramatically.
[32] The US government even designated germanium as a strategic and critical material, calling for a 146 ton (132 tonne) supply in the national defense stockpile in 1987.
[36] Like silicon, gallium, bismuth, antimony, and water, germanium is one of the few substances that expands as it solidifies (i.e. freezes) from the molten state.
Zone refining techniques have led to the production of crystalline germanium for semiconductors that has an impurity of only one part in 1010,[37] making it one of the purest materials ever obtained.
[38] The first semi-metallic material discovered (in 2005) to become a superconductor in the presence of an extremely strong electromagnetic field was an alloy of germanium, uranium, and rhodium.
The growth of these whiskers is one of the primary reasons for the failure of older diodes and transistors made from germanium, as, depending on what they eventually touch, they may lead to an electrical short.
[36] The dioxide, GeO2, can be obtained by roasting germanium disulfide (GeS2), and is a white powder that is only slightly soluble in water but reacts with alkalis to form germanates.
[42] GeS2 forms as a white precipitate when hydrogen sulfide is passed through strongly acid solutions containing Ge(IV).
[48] By heating the disulfide in a current of hydrogen, the monosulfide (GeS) is formed, which sublimes in thin plates of a dark color and metallic luster, and is soluble in solutions of the caustic alkalis.
[42] The first organogermanium compound was synthesized by Winkler in 1887; the reaction of germanium tetrachloride with diethylzinc yielded tetraethylgermane (Ge(C2H5)4).
Organic germanium hydrides such as isobutylgermane ((CH3)2CHCH2GeH3) were found to be less hazardous and may be used as a liquid substitute for toxic germane gas in semiconductor applications.
The s-process is a slow neutron capture of lighter elements inside pulsating red giant stars.
[75] At the end of 2002, the fiber optics industry consumed 60% of the annual germanium use in the United States, but this is less than 10% of worldwide consumption.
Particularly, a very hard special antireflection coating of diamond-like carbon (DLC), refractive index 2.0, is a good match and produces a diamond-hard surface that can withstand much environmental abuse.
In sterling silver alloys, for instance, it reduces firescale, increases tarnish resistance, and improves precipitation hardening.
[32] Semiconductor detectors made of single crystal high-purity germanium can precisely identify radiation sources—for example in airport security.
[92] Due to its use in advanced electronics and optics, Germanium is considered a technology-critical element (by e.g. the European Union), essential to fulfill the green and digital transition.
As China controls 60% of global Germanium production it holds a dominant position over the world's supply chains.
On 3 July 2023 China suddenly imposed restrictions on the exports of germanium (and gallium), ratcheting up trade tensions with Western allies.
Invoking "national security interests," the Chinese Ministry of Commerce informed that companies that intend to sell products containing germanium would need an export licence.
[citation needed] The new dispute opened a new chapter in the increasingly fierce technology race that has pitted the United States, and to a lesser extent Europe, against China.
The US wants its allies to heavily curb, or downright prohibit, advanced electronic components bound to the Chinese market to prevent Beijing from securing global technology supremacy.
[93][94][95] Following China's export restrictions, Russian state-owned company Rostec announced an increase in germanium production to meet domestic demand.
This is primarily because it usually occurs only as a trace element in ores and carbonaceous materials, and the various industrial and electronic applications involve very small quantities that are not likely to be ingested.
[97] U.S. Food and Drug Administration (FDA) research has concluded that inorganic germanium, when used as a nutritional supplement, "presents potential human health hazard".
Soluble inorganic forms of germanium used at first, notably the citrate-lactate salt, resulted in some cases of renal dysfunction, hepatic steatosis, and peripheral neuropathy in individuals using them over a long term.




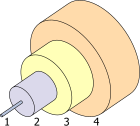
- Core 8 µm
- Cladding 125 µm
- Buffer 250 µm
- Jacket 400 µm

