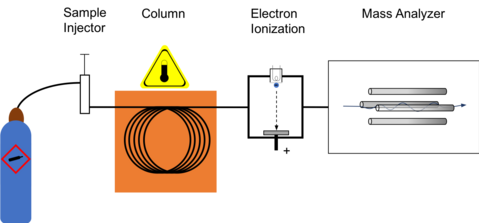
Gas chromatography–mass spectrometry
[4][5] An interest in coupling the methods had been suggested as early as December 1954,[6] but conventional recording techniques had too poor temporal resolution.
[7] The development of affordable and miniaturized computers has helped in the simplification of the use of this instrument, as well as allowed great improvements in the amount of time it takes to analyze a sample.
[8] By 1966 Finnigan and collaborator Mike Uthe's EAI division had sold over 500 quadrupole residual gas-analyzer instruments.
The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (i.e. have the same retention time), which results in two or more molecules that co-elute.
Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer.
Therefore, when an identifying mass spectrum appears at a characteristic retention time in a GC–MS analysis, it typically increases certainty that the analyte of interest is in the sample.
The volatile compounds move into the headspace above the water and are drawn along a pressure gradient (caused by the introduction of the purge gas) out of the chamber.
Additionally one may find a magnetic sector mass spectrometer, however these particular instruments are expensive and bulky and not typically found in high-throughput service laboratories.
Other detectors may be encountered such as time of flight (TOF), tandem quadrupoles (MS-MS) (see below), or in the case of an ion trap MSn where n indicates the number mass spectrometry stages.
This "hard ionization" technique results in the creation of more fragments of low mass-to-charge ratio (m/z) and few, if any, molecules approaching the molecular mass unit.
The molecular fragmentation pattern is dependent upon the electron energy applied to the system, typically 70 eV (electronvolts).
The enhanced molecular ions increase the identification probabilities of both known and unknown compounds, amplify isomer mass spectral effects and enable the use of isotope abundance analysis for the elucidation of elemental formulas.
[20] In chemical ionization (CI) a reagent gas, typically methane or ammonia is introduced into the mass spectrometer.
One of the main benefits of using chemical ionization is that a mass fragment closely corresponding to the molecular weight of the analyte of interest is produced.
[21] In positive chemical ionization (PCI) the reagent gas interacts with the target molecule, most often with a proton exchange.
In negative chemical ionization (NCI) the reagent gas decreases the impact of the free electrons on the target analyte.
A mass spectrometer is typically utilized in one of two ways: full scan or selective ion monitoring (SIM).
When the amount of information collected about the ions in a given gas chromatographic peak decreases, the sensitivity of the analysis increases.
When collecting data in the full scan mode, a target range of mass fragments is determined and put into the instrument's method.
A MS should not be set to look for mass fragments too low or else one may detect air (found as m/z 28 due to nitrogen), carbon dioxide (m/z 44) or other possible interference.
To additionally confirm the likelihood of a potentially positive result, it is relatively important to be sure that the ion ratios of the various mass fragments are comparable to a known reference standard.
[1] A simple and selective GC–MS method for detecting marijuana usage was recently developed by the Robert Koch Institute in Germany.
[23] GC–MS is also commonly used in forensic toxicology to find drugs and/or poisons in biological specimens of suspects, victims, or the deceased.
[24] GC–MS is the main tool used in sports anti-doping laboratories to test athletes' urine samples for prohibited performance-enhancing drugs, for example anabolic steroids.
There are only three manufacturers certified by the FAA to provide these systems,[citation needed] one of which is Thermo Detection (formerly Thermedics), which produces the EGIS, a GC–MS-based line of explosives detectors.
As part of the post-September 11 drive towards increased capability in homeland security and public health preparedness, traditional GC–MS units with transmission quadrupole mass spectrometers, as well as those with cylindrical ion trap (CIT-MS) and toroidal ion trap (T-ITMS) mass spectrometers have been modified for field portability and near real-time detection of chemical warfare agents (CWA) such as sarin, soman, and VX.
[27] Additionally, the systems are smaller, and more mobile, including units that are mounted in mobile analytical laboratories (MAL), such as those used by the United States Marine Corps Chemical and Biological Incident Response Force MAL and other similar laboratories, and systems that are hand-carried by two-person teams or individuals, much ado to the smaller mass detectors.
[28] Depending on the system, the analytes can be introduced via liquid injection, desorbed from sorbent tubes through a thermal desorption process, or with solid-phase micro extraction (SPME).
[33] The MSL Curiosity rover's Sample analysis at Mars (SAM) instrument contains both a gas chromatograph and quadrupole mass spectrometer that can be used in tandem as a GC–MS.
This is increasingly becoming a common way to diagnose IEM for earlier diagnosis and institution of treatment eventually leading to a better outcome.