Ion source
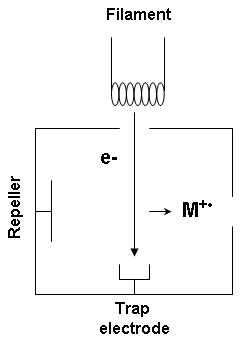
Inside the ion source, the reagent gas is present in large excess compared to the analyte.
Associative ionization is a gas phase reaction in which two atoms or molecules interact to form a single product ion.
Such a separation (together with an appropriate gas-flow scheme) may help reduce the negative effect, that particles released from a processed substrate may have on the plasma chemistry of the gas phase.
[32] A closed drift ion source uses a radial magnetic field in an annular cavity in order to confine electrons for ionizing a gas.
Resonance-enhanced multiphoton ionization (REMPI) is a form of MPI in which one or more of the photons accesses a bound-bound transition that is resonant in the atom or molecule being ionized.
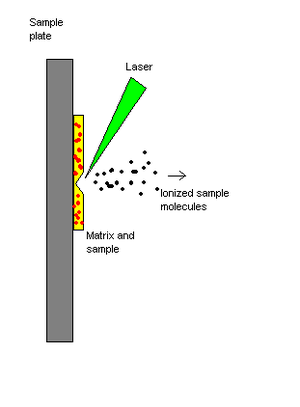
In FAB the analytes is mixed with a non-volatile chemical protection environment called a matrix and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms.
The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm.
[41] Surface-enhanced laser desorption/ionization (SELDI) is a variant of MALDI that is used for the analysis of protein mixtures that uses a target modified to achieve biochemical affinity with the analyte compound.
Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI.
[51] Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity.
Matrix-Assisted Ionization (MAI) is similar to MALDI in sample preparation, but a laser is not required to convert analyte molecules included in a matrix compound into gas-phase ions.
In MAI, analyte ions have charge states similar to electrospray ionization but obtained from a solid matrix rather than a solvent.
Simply introducing the matrix-analyte sample to the inlet aperture of an atmospheric pressure ionization mass spectrometer produces abundant ions.
[54] A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen and the entire aerosol cloud is subjected to a corona discharge that creates ions with the evaporated solvent acting as the chemical ionization reagent gas.
Because like charges repel, the liquid pushes itself out of the capillary and forms an aerosol, a mist of small droplets about 10 μm across.
[59] Contactless atmospheric pressure ionization is a technique used for analysis of liquid and solid samples by mass spectrometry.
Thus, the technique provides a facile means for analyzing chemical compounds by mass spectrometry at atmospheric pressure.
Sonic spray ionization is method for creating ions from a liquid solution, for example, a mixture of methanol and water.
[62] Sonic spray ionization has been coupled with high performance liquid chromatography for the analysis of drugs.
[68] Ultrasonication-assisted spray ionization (UASI) is similar to the above techniques but uses an ultrasonic transducer to achieve atomization of the material and generate ions.
To generate positive ions, the atomic species should have a low ionization energy, and the surface should have a high work function.
This technique is most suitable for alkali atoms (Li, Na, K, Rb, Cs) which have low ionization energies and are easily evaporated.
[71] To generate negative ions, the atomic species should have a high electron affinity, and the surface should have a low work function.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface.
Desorption electrospray ionization (DESI) creates charged droplets that are directed at a solid sample a few millimeters to a few centimeters away.
[77] Plasma-based ambient ionization is based on an electrical discharge in a flowing gas that produces metastable atoms and molecules and reactive ions.
A direct analysis in real time (DART) source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or "metastables").
Excited states are typically formed in the DART source by creating a glow discharge in a chamber through which the gas flows.
ECR ion sources are used as injectors into linear accelerators, Van-de-Graaff generators or cyclotrons in nuclear and elementary particle physics.
This voltage, combined with the high magnetic field between the tips of the internal and external cathodes allow a plasma to start.