Gel electrophoresis of nucleic acids
Gel electrophoresis of nucleic acids is an analytical technique to separate DNA or RNA fragments by size and reactivity.
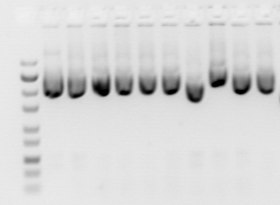
Gels have conventionally been run in a "slab" format such as that shown in the figure, but capillary electrophoresis has become important for applications such as high-throughput DNA sequencing.
[1] Similarly, RNA and single-stranded DNA can be run and visualised by PAGE gels containing denaturing agents such as urea.
A number of factors can affect the migration of nucleic acids: the dimension of the gel pores, the voltage used, the ionic strength of the buffer, and the concentration intercalating dye such as ethidium bromide if used during electrophoresis.
Double-stranded DNA moves at a rate that is approximately inversely proportional to the logarithm of the number of base pairs.
This relationship however breaks down with very large DNA fragments and it is not possible to separate them using standard agarose gel electrophoresis.
[11] This phenomenon can result in band inversion whereby larger DNA fragments move faster than smaller ones in PFGE.
[13] A globular protein or a random coil DNA moves through the connected pores large enough to accommodate its passage, and the movement of larger molecules is more likely to be impeded and slowed down by collisions with the gel matrix, and the molecules of different sizes can therefore be separated in this process of sieving.
In the fully biased mode, the mobility reached a saturation point and DNA beyond a certain size cannot be separated.
[13] Perfect parallel alignment of the chain with the field however is not observed in practice as that would mean the same mobility for long and short molecules.
The orientation of the DNA is progressively built up by reptation after the onset of a field, and the time it reached the steady state velocity is dependent on the size of the molecule.
Real-time fluorescence microscopy of stained molecules showed more subtle dynamics during electrophoresis, with the DNA showing considerable elasticity as it alternately stretching in the direction of the applied field and then contracting into a ball, or becoming hooked into a U-shape when it gets caught on the polymer fibres.
[18] The most common dye used to make DNA or RNA bands visible for agarose gel electrophoresis is ethidium bromide, usually abbreviated as EtBr.
EtBr is a known mutagen,[19] and safer alternatives are available, such as GelRed, produced by Biotium, which binds to the minor groove.
It is more expensive, but 25 times more sensitive, and possibly safer than EtBr, though there is no data addressing its mutagenicity or toxicity in humans.
Visualization can also be achieved by transferring DNA after SDS-PAGE to a nitrocellulose membrane followed by exposure to a hybridization probe.
Shorter wavelength UV radiations (302 or 312 nm) cause greater damage, for example exposure for as little as 45 seconds can significantly reduce transformation efficiency.
Addition of Cytidine or guanosine to the electrophoresis buffer at 1 mM concentration may protect the DNA from damage.
[23] Alternatively, a blue light excitation source with a blue-excitable stain such as SYBR Green or GelGreen may be used.
Gel electrophoresis research often takes advantage of software-based image analysis tools, such as ImageJ.