Glass electrode
The voltage of the glass electrode, relative to some reference value, is sensitive to changes in the activity of a certain type of ions.
[1] In 1909, S. P. L. Sørensen introduced the concept of pH, and in the same year F. Haber and Z. Klemensiewicz reported results of their research on the glass electrode in The Society of Chemistry in Karlsruhe.
There are also specialized ion-sensitive glass electrodes used for the determination of the concentration of lithium, sodium, ammonium, and other ions.
Glass electrodes find a wide diversity of uses in a large range of applications including research labs, control of industrial processes, analysis of foods and cosmetics, monitoring of environmental pollution, or soil acidity measurements... .
[7] For long-term in situ measurements, it is critical to minimize the KCl leak from the reference electrode compartment (Ag / AgCl / KCl 3 M), and to use glycerol-free electrodes[8] to avoid fuelling microbial growth, and to prevent unexpected but severe perturbations related to bacterial activity (pH decrease due to sulfate-reducing bacteria, or even methanogen bacteria).
[citation needed] There are two main types of glass-forming systems:[citation needed] Because of the ion-exchange nature of the glass membrane, it is possible for some other ions to concurrently interact with ion-exchange sites of the glass, and distort the linear dependence of the measured electrode potential on pH or other electrode functions.
For example, some silicate pPNA[clarification needed] electrodes can be changed to pAg function by soaking in a silver salt solution.
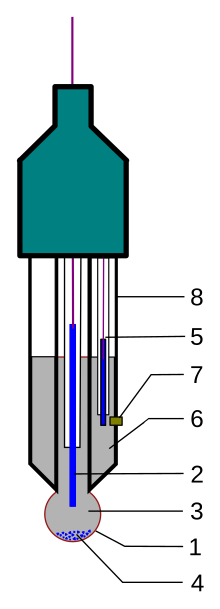
This device is essentially a galvanic cell that can be schematically represented as: The double "pipe symbols" (||) indicate diffusive barriers – the glass membrane and the ceramic junction.
By using the same electrodes on the left and right, any potentials generated at the interfaces cancel each other (in principle), resulting in the system voltage being dependent only on the interaction of the glass membrane and the test solution.
The measuring part of the electrode, the glass bulb on the bottom, is coated both inside and out with a ~10 nm layer of a hydrated gel.
These effects are masked when the electrode is calibrated against buffer solutions but deviations from ideal response are easily observed by means of a Gran plot.
It is necessary to prevent the glass membrane from drying out because the performance is dependent on the existence of a hydrated layer, which forms slowly.