Silver chloride
[4]: 32 Silver-based photographic films were first made in 1727 by Johann Heinrich Schulze with silver nitrate.
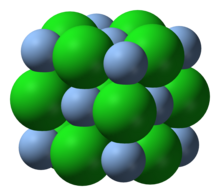
[4]: 38–39 [9] The solid adopts the fcc NaCl structure, in which each Ag+ ion is surrounded by an octahedron of six chloride ligands.
[11][failed verification] Above 7.5 GPa, silver chloride transitions into a monoclinic KOH phase.
[2] AgCl dissolves in solutions containing ligands such as chloride, cyanide, triphenylphosphine, thiosulfate, thiocyanate and ammonia.
Cyanidation produces the soluble dicyanoargentate complex, which is later turned back to silver by reduction.
[4]: 42 Most complexes derived from AgCl are two-, three-, and, in rare cases, four-coordinate, adopting linear, trigonal planar, and tetrahedral coordination geometries, respectively.
[13] In one of the most famous reactions in chemistry, the addition of colorless aqueous silver nitrate to an equally colorless solution of sodium chloride produces an opaque white precipitate of AgCl:[14] This conversion is a common test for the presence of chloride in solution.
Interfering ions for this test are bromide and iodide, as well as a variety of ligands (see silver halide).
[1][4]: 46 AgCl quickly darkens on exposure to light by disintegrating into elemental chlorine and metallic silver.
This reaction is used in photography and film and is the following:[5] The process is not reversible because the silver atom liberated is typically found at a crystal defect or an impurity site so that the electron's energy is lowered enough that it is "trapped".
Even though color photography uses silver chloride, it only works as a mediator for transforming light into organic image dyes.
[21][22] Silver chloride's low solubility makes it a useful addition to pottery glazes for the production of "Inglaze lustre".
[25] This mineral is a source of silver and is leached by cyanidation, where it will produce the soluble [Ag(CN)2]– complex.
[4]: 26 According to the ECHA, silver chloride may damage the unborn child, is very toxic to aquatic life with long lasting effects and may be corrosive to metals.